Several of the currently approved COVID-19 vaccines utilize messenger RNA (mRNA) technology to encode for the production of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike proteins.
Most reports indicate that these vaccines strongly induce the production of antibodies towards the spike protein, particularly the receptor-binding domain (RBD), following the two doses usually prescribed. However, the involvement of CD4+ and CD8+ memory T cells, essential to inducing long-lasting immunity, has been poorly studied.
In a paper recently uploaded to the preprint server bioRxiv* by Tauzin et al. (March 18th, 2021) the ability of a single dose of mRNA vaccine to induce humoral and T cell responses against mutated variants are demonstrated and found to be reinforced in individuals with a history of COVID-19.
How was the study performed?
Sixteen individuals that had not previously been infected with SARS-CoV-2, and an additional sixteen that had been infected around nine months earlier, were involved in the study. Blood was collected from each individual both before receiving the mRNA vaccine and 21 days later, and the presence of RBD-specific antibodies was quantified.
Within the SARS-CoV-2 naïve group, RBD specific antibodies were not detected in serum before receiving the vaccine, while at the second time point all but one bore significantly elevated anti-spike protein Immunoglobulin A (IgA) and Immunoglobulin G (IgG), and mildly elevated Immunoglobulin M (IgM). Having received the vaccine, similarly elevated IgA and IgG levels were observed in both groups, though in this case, IgM was less notably increased in the SARS-CoV-2 experienced individuals.
Total Ig levels towards the RBD or spike protein were elevated in SARS-CoV-2 experienced individuals prior to receiving the vaccine, explaining the less notable increase in IgM levels observed. However, it should be noted that pre-vaccine IgM levels in these individuals were, on average, higher than post-vaccine IgM levels in SARS-CoV-2 naïve individuals.
Cells were transfected with plasmids expressing a variety of known SARS-CoV-2 spike protein mutants alongside spike proteins from other similar coronaviruses. Plasma samples from the 32 participants were presented to the cells. As expected, the pre-vaccine naïve group did not contain any anti-spike antibodies, while the experienced group, and following vaccination, both groups, did. Finally, to evaluate whether the vaccine response was limited to the RBD of the spike protein ELISA assays were performed to measure anti-full spike protein antibody levels. It was noted that after vaccination, the SARS-CoV-2 naïve group elicited antibodies to similar levels as those observed in SARS-CoV-2 experienced individuals prior to receiving the vaccine.
Vaccinated SARS-CoV-2 naïve individuals were able to recognize fewer variants of the spike protein compared with SARS-CoV-2 experienced individuals, both before and after vaccination. However, vaccinating the latter group did further boost immunity towards the same SARS-CoV-2 spike proteins over the already higher baseline level.
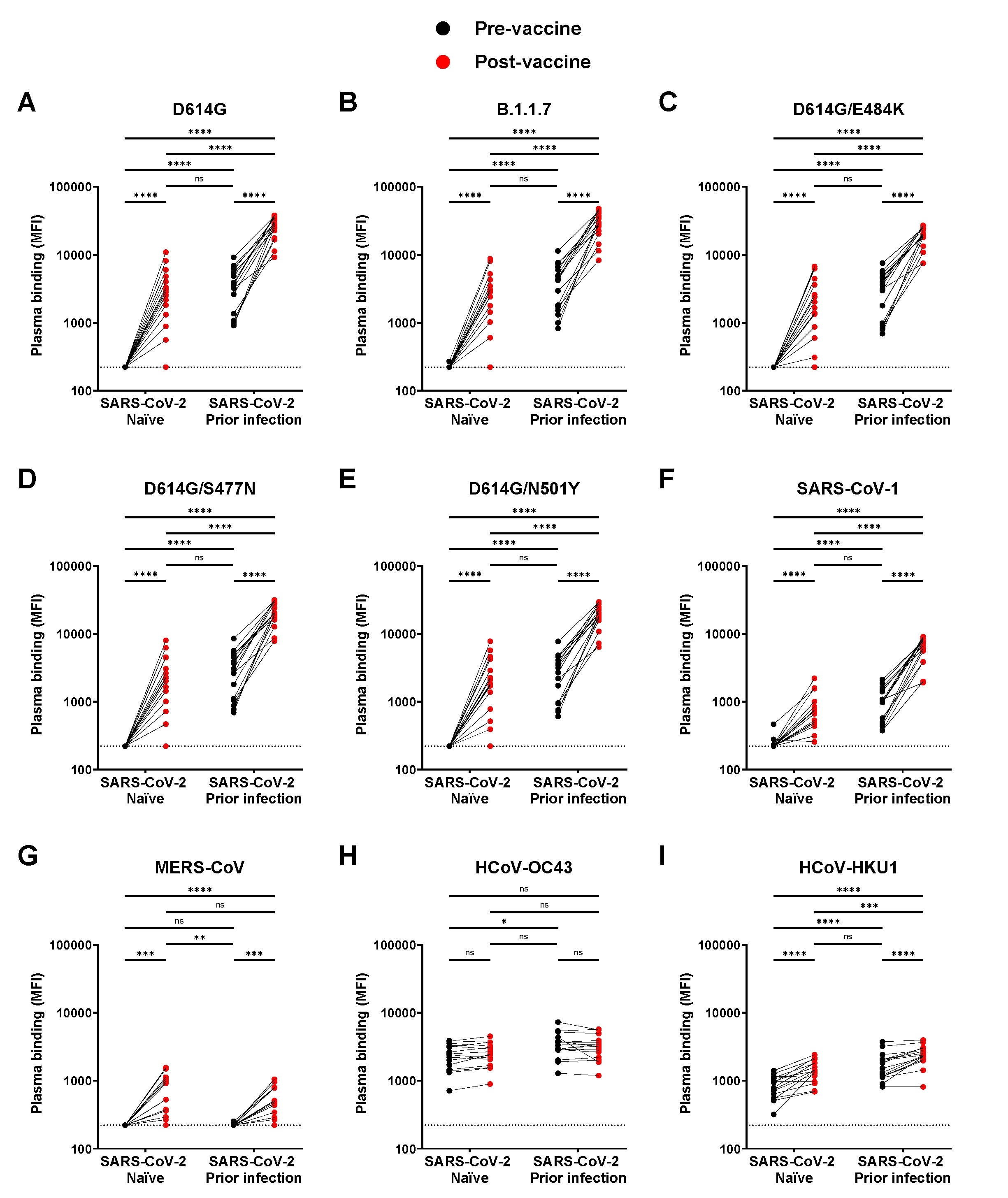
Detection of SARS-CoV-2 Spike variants and other Betacoronaviruses. (A-I) Cell-surface staining of 293T cells expressing full-length Spike from different SARS-CoV-2 variants and other human Betacoronavirus using plasma samples collected before and after first dose of vaccination in SARS-CoV-2 naïve and previously-infected donors. The graphs represent the median fluorescence intensities (MFI) obtained. Limits of detection are plotted. (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001; ns, non-significant)

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Is the vaccine efficacious?
The quantity of anti-SARS-CoV-2 antibodies in plasma returns to baseline levels relatively quickly in hosts following viral challenge. Indeed, the presence of neutralization antibodies was not observed to significantly increase in naïve individuals post-vaccination.
However, antibody-dependent cellular cytotoxicity (ADCC ) was noted to increase significantly following vaccination and remain high.
Fc receptors are found on the surface of various immune cells and bind to antibodies that are attached to pathogens, stimulating phagocytosis or ADCC, and play an important role in immunity. As with the presence of anti-spike protein antibodies, SARS-CoV-2 naïve individuals demonstrated no ADCC activity prior to vaccination but exhibited a significant increase following a single dose, reaching comparable levels to those in pre-vaccinated SARS-CoV-2 experienced individuals.
T cells depend upon antibodies bonding with the relevant target with high specificity, and thus there is often a correlation of efficacy between the two. IgA, IgG, and CD4+ T cell responses were seen to rise correlatively following vaccination, while IgM levels rise only mildly. The improved responses observed in SARS-CoV-2 experienced individuals compared to the naïve group suggests that having pre-existing CD4+ T cell responses is beneficial to the development of effective humoral response towards SARS-CoV-2, and these defenses can be bolstered further by mRNA vaccine boosters.
Following vaccination, a significant increase in CD4+ T cell responses was noted, particularly in those that had been infected with SARS-CoV-2 before. CD8+ T cell response was also significantly increased following vaccination, but only in the SARS-CoV-2 experienced group, with the naïve group showing only a mild increase in activity. SARS-CoV-2 naïve individuals exhibited a more significant increase in IgG levels than IgM or IgA. Overall, vaccination induced strikingly similar responses in naïve individuals as past SARS-CoV-2 infection had in others prior to vaccination. SARS-CoV-2 specific T cell immune response was noted following vaccination in both groups, though more significantly so in the experienced group, suggesting that naïve individuals would benefit more greatly from the usually prescribed two doses of vaccine.
In this study, the Pfizer-BioNTech mRNA vaccine applied was found to be up to 90% efficacious following only a single dose two weeks after administration.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses, Alexandra Tauzin, Manon Nayrac, Mehdi Benlarbi, Shang Yu Gong, Romain Gasser, Guillaume Beaudoin-Bussières, Nathalie Brassard, Annemarie Laumaea, Dani Vézina, Jérémie Prévost, Sai Priya Anand, Catherine Bourassa, Gabrielle Gendron-Lepage, Halima Medjahed, Guillaume Goyette, Julia Niessl, Olivier Tastet, Laurie Gokool, Chantal Morrisseau, Pascale Arlotto, Leonidas Stamatatos, Andrew T. McGuire, Catherine Larochelle, Pradeep Uchil, Maolin Lu, Walther Mothes, Gaston De Serres, Sandrine Moreira, Michel Roger, Jonathan Richard, Valérie Martel-Laferrière, Ralf Duerr, Cécile Tremblay, Daniel E. Kaufmann, Andrés Finzi, bioRxiv, 2021.03.18.435972; doi: https://doi.org/10.1101/2021.03.18.435972, https://www.biorxiv.org/content/10.1101/2021.03.18.435972v1
- Peer reviewed and published scientific report.
Tauzin, Alexandra, Manon Nayrac, Mehdi Benlarbi, Shang Yu Gong, Romain Gasser, Guillaume Beaudoin-Bussières, Nathalie Brassard, et al. 2021. “A Single Dose of the SARS-CoV-2 Vaccine BNT162b2 Elicits Fc-Mediated Antibody Effector Functions and T Cell Responses.” Cell Host & Microbe 29 (7): 1137-1150.e6. https://doi.org/10.1016/j.chom.2021.06.001. https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(21)00279-1.