A Korea-based company EyeGene Inc. has recently developed a liposome-based mRNA candidate vaccine against coronavirus disease 2019 (COVID-19), which contains full-length spike protein of the European variant (D614G) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a vaccine antigen.
The antigen has been modified with 2P-3Q substitution to make it more stable and resistant to host cell proteases. The preliminary animal experimentations reveal that the vaccine candidate (EG-COVID) is highly effective in triggering both antibody-mediated and cell-mediated host immune responses. The study is currently available on the bioRxiv* preprint server.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
As of March 23, 2021, globally, there have been more than 122 million confirmed cases of COVID-19, including 2.7 million deaths, reported to the World Health Organization (WHO). As a global attempt to achieve herd immunity and control the spread of SARS-CoV-2, several potential vaccines have been developed at record speed. Some of these vaccines have shown good efficacy and safety levels in human clinical trials and are currently rolling out in many countries. According to the WHO report, more than 397 million doses of COVID-19 vaccines have already been administered globally.
In the current study, the scientists have described the anti-SARS-CoV-2 effectiveness of an mRNA-based candidate vaccine, namely EG-COVID, developed by a Korea-based company EyeGene Inc. The full-length spike protein of the European SARS-CoV-2 variant (D614G) was modified at the S1/S2 polybasic cleavage site along with 2 proline substitution at residues K986 and V987 in the S2 fusion region (2P-3Q substitutions) has been used as a vaccine antigen. The modifications have been made to increase protein stability and prevent proteolytic activation of the spike protein.
The scientists have used a cationic liposome (positively charged)-based vaccine delivery system that can effectively hold and deliver negatively charged vaccine mRNA to cells and tissues.
Important observations
The scientists developed both liquid and lyophilized forms of EG-COVID and verified the structural stability and functionality of the constructs. Using cryo-transmission electron microscopy, they observed that both liquid and lyophilized EG-COVID formed small unilamellar liposomal vesicles (diameter: 80 – 100 nm).
To determine the vaccine mRNA delivery ability of cationic liposomes, they intramuscularly injected the mice with cationic liposome-mRNA complexes and observed that the liposomes delivered the mRNA as effectively as lipid nanoparticles. By conducting a series of in vitro experiments, they confirmed that after its cellular delivery, EG-COVID is capable of expressing the SARS-CoV-2 spike protein. For these experiments, they used lyophilized forms of several EG-COVID candidates that differed in their lipid compositions. Interestingly, the findings revealed that the lipid composition of EG-COVID could influence the cellular expression of the spike protein.
EG-COVID induces robust humoral and cellular immune responses
To examine the vaccine efficacy, they intramuscularly injected the mice with both liquid and lyophilized forms of EG-COVID twice at an interval of 3 weeks. Two weeks after administration of the second dose, they estimated the titers of anti-receptor binding domain (RBD) antibodies and the levels of splenocyte-secreted interferons (IFN-g). Furthermore, they investigated whether EG-COVID-induced antibodies can effectively neutralize SARS-CoV-2.
The findings of in vivo experiments revealed that the 2-dose regimen of EG-COVID significantly induced the production of both anti-RBD binding antibodies and SARS-CoV-2 neutralizing antibodies in mice. Moreover, increased secretion of IFN-g from splenocytes was observed in EG-COVID-immunized mice. Importantly, the serum samples obtained from immunized mice were found to inhibit the infection of VERO cells by the original Wuhan strain of SARS-CoV-2.
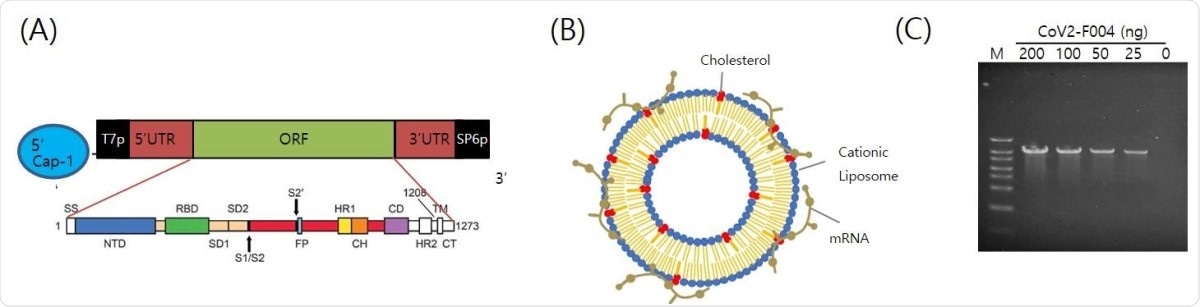
The structure of EG-COVID vaccine (A) A schematic illustration of antigen coding mRNA Cov2-F004 structure. (B) Schematic illustration of the structure of EG-COVID. (C) The functionality of CoV2-F004 Structure. In vitro transcription of CoV2-F004 showed the increase of transcript according to CoV2-F004 amount used in the transcription. M; size marker.
Study significance
The study reveals that EG-COVID, an mRNA-based COVID-19 candidate vaccine, can inducing robust humoral and cellular immune responses in mice. Since full-length spike protein of the European SARS-CoV-2 variant containing D614G mutation has been used as a vaccine antigen, EG-COVID is expected to be effective against newly emerging viral variants.
The advantage of the lyophilized form of EG-COVID is that it can be stored and transported easily without requiring a special cold chain. This may ensure a wide-spread and timely distribution of the vaccine, especially in developing countries.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.