In a methodologically ‘state-of-the-art’ approach, US researchers have developed a protein-based nanoparticle vaccine against coronavirus disease (COVID-19), with a swift generation of antibodies and the potential to protect individuals who cannot receive other COVID-19 vaccines for medical reasons. The research is posted to the bioRxiv* preprint server.
Vaccines currently in use against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a causative agent of COVID-19, focus on the viral spike glycoprotein that mediates cell entry by targeting angiotensin receptor 2 (ACE2) as the main receptor and heparin as the co-receptor.
However, not all vaccine candidates are the same, and those with a multivalent display of antigen are able to induce a longer-lasting immune response when compared to monovalent antigens. Such multivalent options tend to use virus-like particles or nanoparticles as their principal strategy for vaccine development.
In any case, we are familiar with the footprint of the most potent neutralizing antibodies that usually line within the epitope for ACE2 binding on the receptor-binding domain of the spike glycoprotein, suggesting that this is actually a desirable place on the virus to exert neutralizing action.
Having all that in mind, a research group led by Dr. Maggie Gu from the ReVacc Scientific biotech company in Maryland, United States, developed a nanoparticle vaccine candidate named REVC-128, and showed its potential for enabling early protection against SARS-CoV-2.
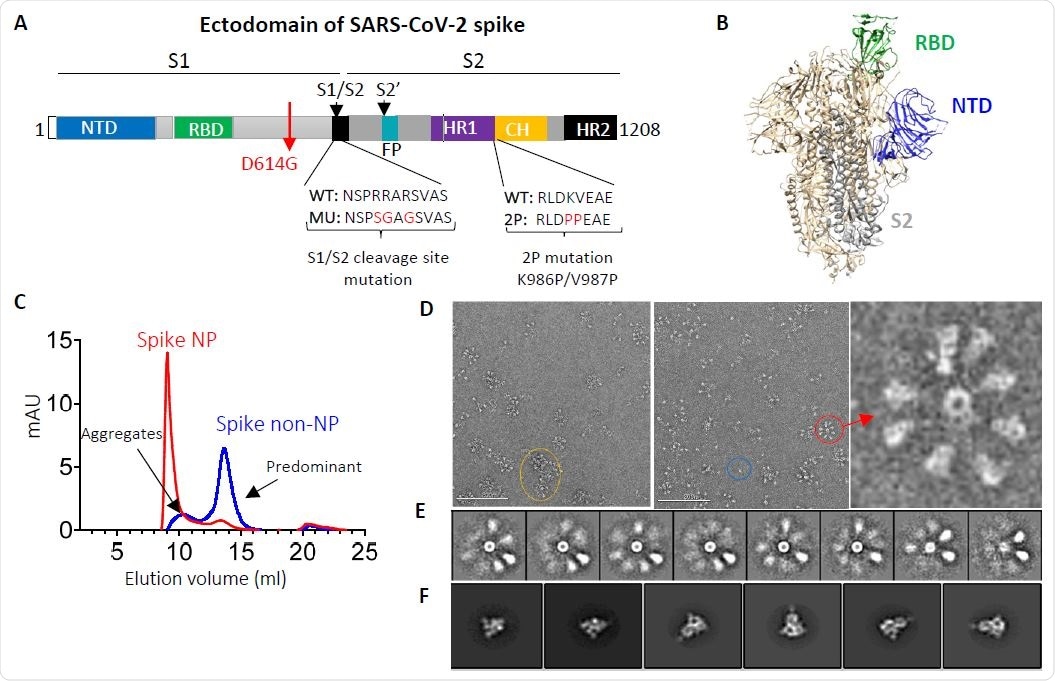
SARS-CoV-2 spike ectodomain and nanoparticle presenting trimeric spike ectodomain. (A) Schematic of SARS-CoV-2 spike protein ectodomain. NTD: N-terminal domain; RBD: receptor-binding domain; S1/S2= S1/S2 protease cleavage site; FP= fusion peptide; HR= heptad repeat. Two arrows indicate the cleavage sites. The native furin cleavage site was knocked out (RRAR→SGAG), two proline at positions K986 and V987 substituted, and one glycine at position D614 substituted for ectodomain expression and nanoparticle conjugation. (B) Schematic of prefusion conformation of SARS-Cov-2 trimeric S structure with NTD, RBD and S2 subunit highlighted in blue, green and grey on one protomer, respectively (PDB:6VSB). (C) Size-exclusion chromatography (SEC) profiles of spike NP (red) and spike non-NP (blue) presentation on a Superose 6 column. (D) Spike NP observation by negative stain EM. In the raw micrograph, the representative of nanoparticle single particle, spike NP aggregates and NPs with varying stoichiometries was circled in blue, yellow and red, respectively. The closer observation of a selected multivalent spike NP is on the right. The grey scale bar represents 200 nm. (E) 2D classes averages of spike NP. The pictures show varying numbers of spike proteins on NPs. (F) Spike trimers are in the desired prefusion conformation on NP.
From structure generation to animal experiments
In a nutshell, multiple trimeric subunits of spike glycoprotein ectodomain with glycine at position 614 were multimerized onto the ferritin nanoparticle in order to develop this vaccine candidate, and the design aimed at preferentially presenting the neutralizing antibody epitope.
Furthermore, in order to evaluate the immunogenicity of this vaccine, two groups of laboratory mice were immunized with spike nanoparticle and spike non-nanoparticle with the Sigma Adjuvant System by utilizing a subcutaneous injection route, while the third group of mice was given phosphate-buffered saline as a negative control.
Vaccine protection efficacy was then evaluated in detail in Syrian golden hamsters, which one of few small animal models susceptible to the infection with the SARS-CoV-2 virus. The scientists assessed not only clinical and immunological correlates of infections but also conducted histopathology analyses of the lungs and nares of two groups of infected hamsters.
High titers and thermostability
In vitro characterization of REVC-128 confirmed its antigenic and structural integrity, while subsequent immunogenicity evaluation in mice implied that a single dose of this vaccine could induce potent serum neutralizing antibody titers two weeks after immunization.
This is significantly higher than titers induced by trimeric spike protein without nanoparticle presentation. One really favorable trait revealed by this study is a significant thermostability of this new vaccine candidate – up to 37 °C for at least four weeks.
Furthermore, the results demonstrate that vaccination successfully protects hamsters against SARS-CoV-2 viral challenge with evidence of suppressed viral loads, steady body weight, and the reduction of tissue damage (lung and nares) for protected animals, in comparison to higher viral loads, weight loss, and tissue damage in unprotected ones.
A promising vaccine candidate
“These findings, along with a long history of safety for protein vaccines, suggest that the REVC-128 is a safe, stable and efficacious single-shot vaccine candidate to induce the earliest protection against SARS-CoV-2 infection”, summarize study authors the main findings of this bioRxiv paper.
This is actually the first COVID-19 vaccine that exerts vaccine-induced protection two weeks after immunization, which is much earlier compared to other vaccine candidates that show induced protection approximately four weeks after the first dose of immunization.
In addition, this type of vaccine design might prevent the development of severe symptoms in cases when patients get infected with other coronaviruses after immunization, such as seasonal coronaviruses (which are one group of common cold viruses) mutated SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gu, M. et al. (2021). One dose of COVID-19 nanoparticle vaccine REVC-128 provides protection against SARS-CoV-2 challenge at two weeks post immunization. bioRxiv. https://doi.org/10.1101/2021.04.02.438218, https://www.biorxiv.org/content/10.1101/2021.04.02.438218v1
- Peer reviewed and published scientific report.
Gu, Maggie, Jonathan L. Torres, Yijia Li, Alex Van Ry, Jack Greenhouse, Shannon Wallace, Chi-I Chiang, et al. 2021. “One Dose of COVID-19 Nanoparticle Vaccine REVC-128 Protects against SARS-CoV-2 Challenge at Two Weeks Post-Immunization.” Emerging Microbes & Infections 10 (1): 2016–29. https://doi.org/10.1080/22221751.2021.1994354. https://www.tandfonline.com/doi/full/10.1080/22221751.2021.1994354.