With the emergence of several variants of concern (VOCs) of the virus that is responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic, matters threaten to take a new turn that further jeopardizes the chances of getting a grip on this outbreak. This is because these variants have shown considerable resistance to natural and vaccine-induced antibodies, as well as several therapeutic antibodies, produced against the wildtype or Wuhan variant.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Escape mutations hamper pandemic control by vaccines
This virus, called the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has already caused over 132 million infections, and 2.8 million deaths. Without effective antivirals, and with the infeasibility of long-term non-pharmaceutical interventions (NPIs), vaccines appear to be the only way out.
With the rollout of several vaccines at the beginning of 2021, a collective sense of relief was palpable. However, many questions remain as to the extent and duration of immunity. Recently, several VOCs have been reported, which are neutralized less efficiently compared to the ancestral virus strain.
This is probably due to the presence of several punctate and grouped mutations affecting the viral spike protein. This protein mediates viral attachment to the host cell receptor, the angiotensin-converting enzyme 2 (ACE2), as well as cell entry.
These mutations alter interactions at and near the binding interface, allowing variants to evade host immune responses. The SA variant (501Y.V2) is particularly disturbing, as it displays much stronger resistance to current vaccine-elicited antibodies than other variants due to its collection of mutations in dominant neutralizing epitopes.
In fact, South Africa has already stopped the use of the Astra-Zeneca vaccine due to the lack of efficacy against this variant, which is found at high frequency in this country.
The current scenario resembles a race between vaccine development and the virus, as the latter mutates to escape immune defenses, requiring vaccine updates as well as improved vaccine mobilization to achieve global immunization.
The doctrine of original antigenic sin
The first time a pathogen is encountered by the human body, a pattern is set for future responses to the same microbe, even if it is mutated. This is called immunological imprinting, also called original antigenic sin.
With the influenza A virus, this has been shown to result in a powerful response to the first strain encountered, with lower levels of immune response to later variants. Thus, updated vaccines and regimens must be designed, taking this consideration into account.
If this is the case, the new variants can be efficiently protected against by the use of a heterologous boost, which will call upon cross-protection by memory cells, rather than a homologous two-dose prime-boost protocol.
Study details
The current study was carried out on rhesus macaques. The researchers used two doses of the spike protein of the Wuhan variant, stabilized in the prefusion state, with appropriate adjuvant to enhance its immunogenicity. The two doses were given at an interval of one month.
The prime dose was followed by detectable neutralizing activity against the Wuhan variant, but not the SA variant.
The boost dose resulted in an increase in neutralizing activity against this variant, with a decline over the next few months, as expected from immunization reports. The neutralizing response against the SA variant was nine-fold lower, on average, again corresponding to earlier responses in vaccination programs.
In one of the three macaques, the SA strain showed greater sensitivity to neutralization by this vaccine, however.
Results of a heterologous boost
The same macaques were given a second boost dose at six months from the first, using RBD from the SA variant, at three dosages – 2, 10 and 50 μg, all in adjuvanted formulations. Only two macaques were left at the end of the study as one developed an unrelated illness and was terminated five days into the second phase of the study.
After two weeks, the other two animals showed neutralizing responses to both variants, at a geometric mean titer (GMT) of neutralizing antibody around 12,000 for both. In comparison, the GMT against the Wuhan variant with the first boost dose was about 4,000.
The ability to efficiently neutralize the SA variant was unique to this protocol. Earlier experiments using three doses of Wuhan variant spike as the immunizing agent failed to improve the neutralizing response to the SA variant at any point.
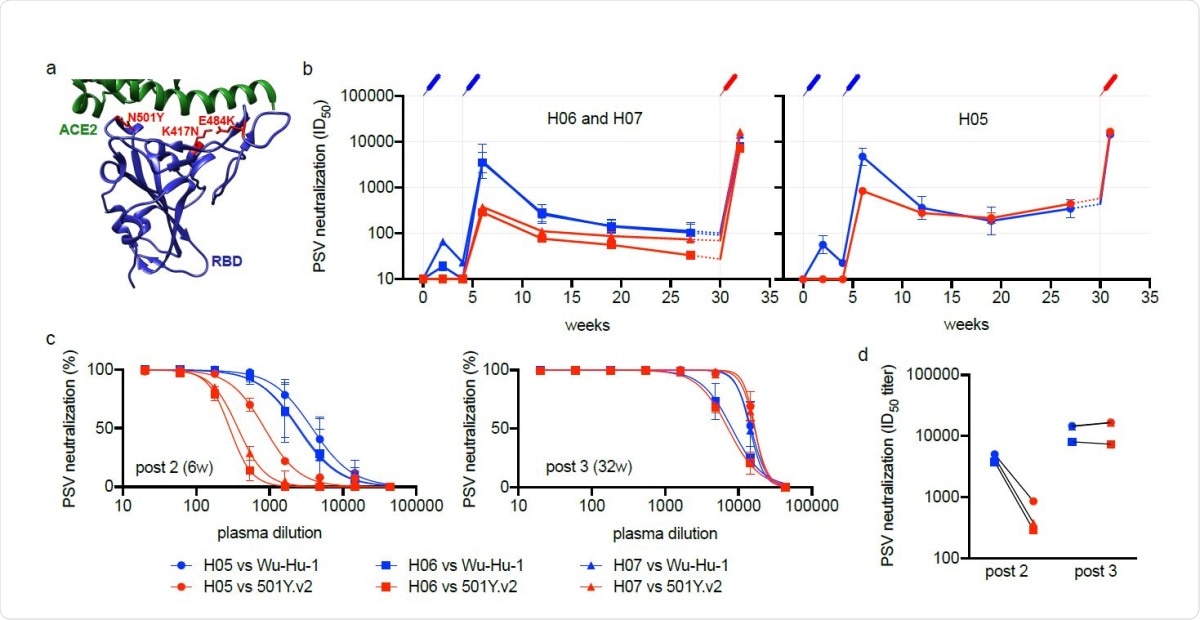
Heterotypic RBD boost drives a potent cross-neutralizing antibody response. (a) Depiction of the RBD immunogen (PDB:6MOJ 18) used as a heterotypic boost in this study, that incorporates the three RBD mutations (located in red) defining lineage 20H/501Y.V2. The cellular receptor, ACE2, is shown in green. (b-d) Neutralizing antibody responses over time to Wu-Hu-1 (blue) and 501Y.V2 (red) pseudotyped viruses (PSV) are shown for three immunized macaques: (b) H06 and H07 (left) and H05 (right), plotted separately as they exhibit different trajectories prior to the heterotypic boost. Syringes indicate the timing of immunizations (blue: Wu-Hu-1 spike at 0 and 4-weeks, red: 501Y.V2 RBD at 30-weeks). Titers from 27-30 weeks (shown with dashed lines) have been extrapolated for clarity. Error bars depict the geometric SD. (c) While neutralization of 501Y.V2 was significantly reduced at 6 weeks, corresponding to peak responses 2 weeks following the second spike dose (left), neutralization was restored following subsequent heterotypic RBD boost (right), such that 501Y.V2 (red) and Wu-Hu-1 (blue) were potently neutralized at similar titers (d) in all three animals.
What are the implications?
The study demonstrates that even with an initial priming dose using the Wuhan spike variant, a heterologous RBD was able to trigger a potent immune recall response, which was stronger than the primary boost dose.
A dose as low as 2 μg of the SA spike RBD was able to elicit this reaction. The role played by the adjuvant in this response is yet to be established. Overall, the researchers have demonstrated that it is possible to produce powerful cross-neutralizing antibody responses, using heterologous virus antigens to boost the earlier immune response.
The RBD is a small protein that is stable in storage and is expressed at high levels. It is capable of being rapidly manufactured. Thus, this study could turn attention to the use of a heterologous RBD or other SARS-CoV-2 immunogens to increase vaccine efficacy.
These data indicate that soluble RBD booster immunizations represent an attractive strategy to broaden vaccine protection from new SARS-CoV-2 variants.”

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.