As the world approaches the grim milestone of three million deaths from COVID-19 disease, a new preprint research paper posted to the bioRxiv* server shows that the presence of gut bacteria in the plasma may be an indicator of progressive disease. In patients with pre-existing comorbidities, COVID-19 is associated with more severe disease.
The gut is a well-established route of infection and target for viral damage by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent for COVID-19. This is supported by the clinical observation that about half of COVID-19 patients exhibit gastrointestinal (GI) symptoms.
Hospitalized patients with critical COVID-19 also often have gut complications. Besides the above, venous or arterial thromboembolism of the mesenteric vessels and small bowel ischemia is reported, especially in patients hospitalized for more extended periods.
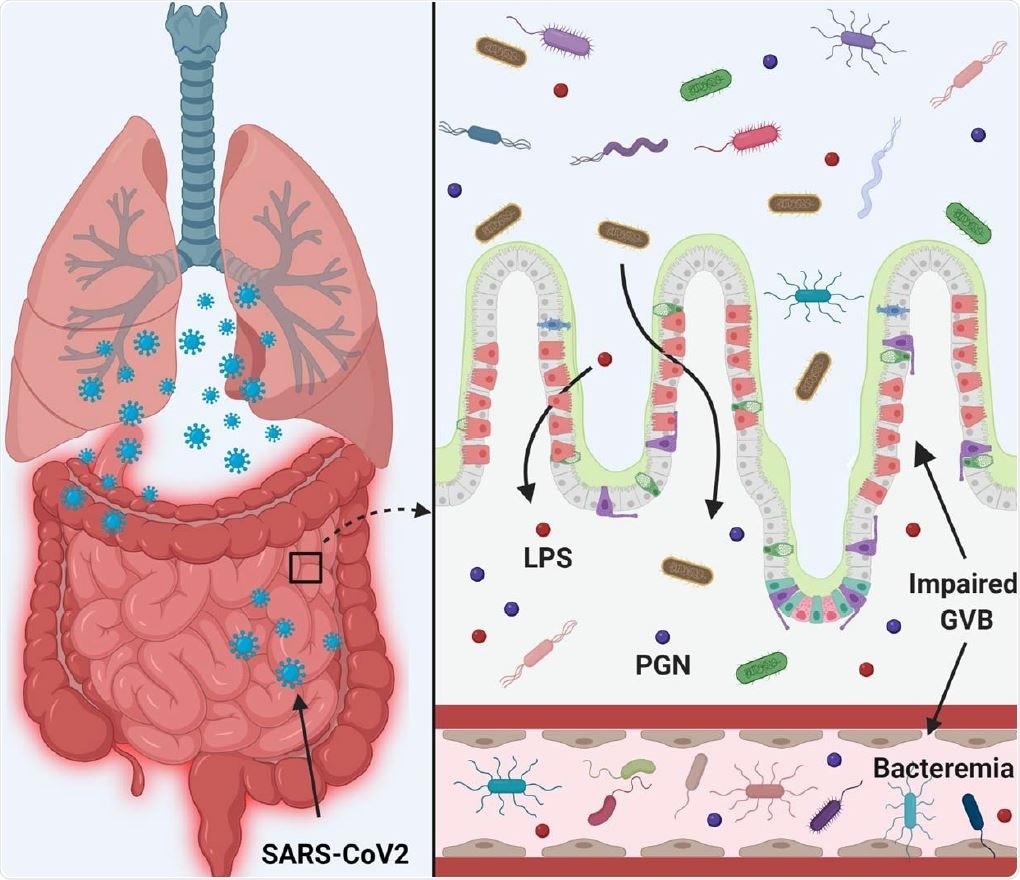
SARS-CoV-2 infection disrupts the gut barrier and leads to elevation of systemic bacterial lipopolysaccharide and peptidoglycan and serves to enhance systemic inflammation. Therefore, leaky gut and microbial dysbiosis could contribute to cytokine storm in patients severely ill with COVID -19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study aims and details
The current study, based in Birmingham, Alabama, in the USA, aimed to capture the presence of plasma biomarkers that indicate breaches of the gut epithelial barrier, and the presence of gut microbes in the plasma.
Unfortunately, both could not be tested in the same subset of patients due to the small volume of plasma available.
Of the 30 patients enrolled in the study with confirmed SARS-CoV-2 infection, all had diarrhea and nausea along with fever and shortness of breath. The median age was 63 years. Only two patients had a critical disease.
Over a third were diabetic, and half experienced blood clots. Of the 30, 23 patients were overweight. Five patients had an in-hospital fatal outcome.
Laboratory results
Lymphopenia and anemia were observed in half, and two-thirds of the patients, along with high monocyte counts. Neutrophils were also elevated, in 60% of males compared to 45% of females. This is significant since they are the first responders to any infection.
Total leukocyte counts were elevated in about 40% of individuals with COVID-19, but platelet count abnormalities were observed in only 17% of subjects. Only two patients showed high levels of brain natriuretic peptide (BNP), probably due to heart failure.
C-reactive peptide, an inflammatory marker, was elevated in all patients, with six patients showing levels consistent with severe inflammation. Eight subjects showed high ferritin levels, with half of them having levels indicative of inflammation.
Almost all the patients had high fasting glucose and lactate dehydrogenase (LDH) levels. About two-thirds had anemia, Increased troponin-I levels, suggesting cardiac injury, were found in 80% of male subjects, vs only one female subject.
Gut microbes in plasma
The 14 plasma samples sent for evaluation for the presence of bacteria yielded over 150,000 sequencing reads, with the signal indicating strong bacterial presence in two-thirds of the samples. The total microbial population was comparable between patients with COVID-19.
Using polymerase chain reaction, a dysbiosis index was arrived at to measure the abundance of bacterial groups in each sample. All the nine samples which indicated the presence of bacteria showed the same three major phyla, Proteobacteria, Firmicutes, and Actinobacteria, with one patient showing unknown bacteria in more significant numbers among all 14.
These are the same that have been found in healthy plasma, as well.
The most enriched phylum was Proteobacteria, while Bacteroides were present in very limited numbers. Among the two patients with a fatal COVID-19 outcome, the number of Firmicutes was low. Perhaps the abundance of this phylum may be a biomarker for severity of disease.
Both Gram-negative bacteria and lipopolysaccharides (LPS), which is a major endotoxin originating from the cell wall of these bacteria, are higher in the plasma samples from COVID-19 patients.
Gut barrier breaches
The presence of gut microbes in plasma may suggest defects in the gut epithelial barrier, allowing bacteria to migrate through the epithelial cells into the systemic blood vessels. This is an important component in systemic inflammation and underlies the progression of COVID-19 in these patients.
As a marker of gut permeability, fatty acid-binding protein-2 (FABP2) levels were measured, as this is a protein found within intestinal epithelial cells to bind free fatty acids, cholesterol, and retinoids. As such, its elevation in plasma indicates mucosal damage in the gut.
As expected, FABP2 levels were high in the plasma of COVID-19 patients relative to healthy individuals.
Gut microbial peptides in plasma are toxic in that they trigger inflammatory pathways and lead to systemic damage. As a measure of this phenomenon, the researchers observed higher levels of peptidoglycan (PGN) and LPS in COVID-19 plasma, at almost double the levels in healthy controls.
What are the implications?
The translocation of gut microbes, normally found only in feces, into the systemic circulation is a fundamental determinant of immune function and metabolism. The presence of gut microbes in plasma may trigger and also exacerbate inflammatory signaling pathways in the body.
Inflammation is key to the pathogenesis of severe and critical COVID-19. This study's findings may support the theory that this is driven by gut bacterial movement into the body's circulation in these patients. This, in turn, could be due to higher gut permeability because of epithelial barrier dysfunction.
Virus shedding in the feces has been found to persist for up to a month after lung symptoms resolve, indicating that viral colonization of the gut may be of longer duration than of the airways.
COVID-19 patients in this sample were more likely to be diabetic and obese relative to the controls. In such patients, the commensal bacteria Lactobacillus are less abundant, and this reduction was found in a small group of nine patients tested at the beginning of hospitalization in this study.
Most COVID-19 deaths are due to sepsis. In this study, the abundance of multiple pathogenic species such as Acinetobacter and Pseudomona was higher in the gut. Even after the infection resolved, dysbiosis persisted, indicating that the gut may suffer the effects of this illness over the long term.
The plasma metabolome is linked to the gut microbiome in the pathogenesis of many diseases. Failure of the gut barrier leads to the detection of bacterial metabolic products in the plasma, in conditions like ulcerative colitis.
The study suggests, "Leaky gut and microbial dysbiosis could contribute to cytokine storm in patients severely ill with COVID -19."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources