The corticosteroid budesonide shows promise in treating patients with mild COVID-19 infection. Interim analysis of the phase 3 PRINCIPLE clinical trial was posted to the medRxiv* preprint server. Researchers found a 3-day faster recovery of COVID-19 symptoms in people at high risk for severe side effects administered a budesonide inhaler. There was also a 2.1% reduction in hospitalization and death.
Corticosteroids such as dexamethasone have shown to be effective in alleviating the severity of COVID-19 infection. Adding budesonide could give doctors more options in treating patients, especially as it is already readily available in many primary care settings and is in the World Health Organization’s List of Essential Medicines. The medicine is also known to be easier to use for unwell, comorbid, and potentially frail older patients.
The research team writes:
“Several randomized trials have demonstrated the benefits of systemic corticosteroids for the treatment of people hospitalized with COVID-19. Our findings are immediately relevant for clinical practice as they suggest that early treatment in the community with inhaled corticosteroids is effective at speeding recovery, which has important benefits for patients and wider society.”
The trial is still ongoing, with the final analysis being made available once patients given inhaled budesonide completed their 28-day follow-up.
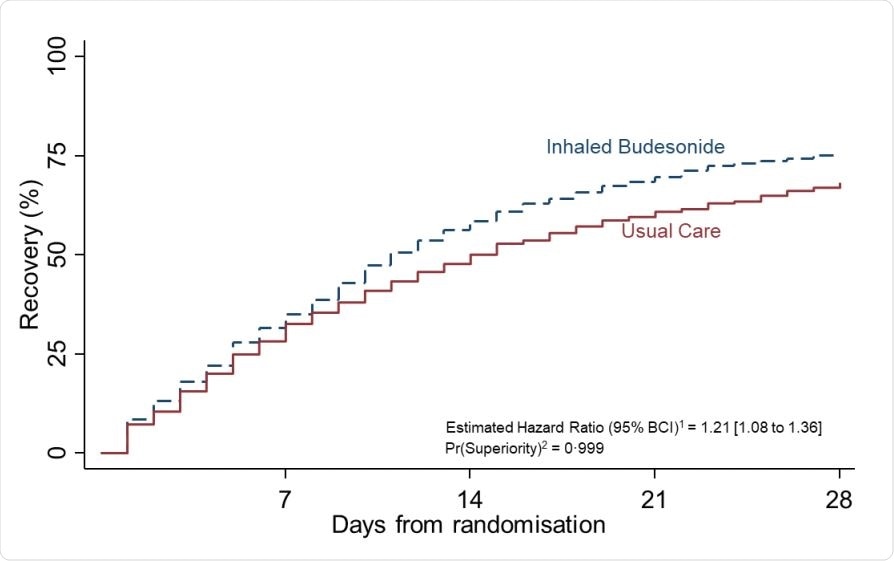
Summary and results of the time to first self-reported recovery SARS-CoV-2 positive analysis population and data extracted on March 25, 2021
Trial Design
The multicenter, open-label, multi-arm PRINCIPLE trial enrolled people over the age of 65 or people over the age of 50 with a history of comorbidities from May 17, 2020, across the United Kingdom. All patients were currently positive for COVID-19 infection within the past 14 days.
Study participants were excluded if they were already taking inhaled or systemic corticosteroids, cannot use an inhaler, or if adding inhaled budesonide would be a contraindication to other medication.
Patients were randomly assigned to either the budesonide, azithromycin, doxycycline or usual care groups. Recently on March 4, 2021, the researchers added a colchicine group.
Enrollment for the budesonide arm began on November 27, 2020, where patients received usual care in addition to 800µg twice daily for 14 days. The patient’s doctor administered the inhaler, or in some cases, the research team would mail the inhaler directly to the patient. Instructions for use were provided via video.
Each patient marked their progress by an online daily symptom diary for 28 days. Researchers also made follow-up phone calls on days 7, 14, and 28.
Patient enrollment stopped on March 31, 2021, because independent committees determined accumulating further data would make it unlikely to change current hospitalization and death results. It would also be unethical to continue given vaccine availability and lower than anticipated event rate.
Details on the budesonide arm
The interim analysis is based on data collected from the start of the trial to March 25, 2021. A total of 4,663 people were enrolled, with 1,032 patients randomly assigned budesonide. About 85.1% had taken a COVID-19 diagnostic test, where 66% were positive.
In terms of the budesonide and usual care arm, the average age was 62.8 years, and 83.2% had comorbidities. The median was about 6 days from symptom onset.
A total of 929 patients gave information on their medication history. About 79.9% of those patients randomly assigned to budesonide had taken budesonide for at least 7 days.
Trial results
Researchers found that patients taking inhaled budesonide had a faster COVID-19 recovery time by 3 days than patients who only received usual care.
People who did follow up in the 28-day follow-up period reported lower hospitalizations in the budesonide group than the usual care group.
Although there isn’t sufficient information to connect budesonide use to a faster recovery time in hospitalizations, the researchers note the probability of superiority was 0.928, which does not meet the predefined superiority threshold of 0.975.
Subjective feelings of well-being were higher with patients taking budesonide than people with usual care.
Two patients in the budesonide group were admitted to the hospital for severe health complications, but the researchers determined hospitalization was not connected to COVID-19 infection.
Based on the results, the researchers suggest the current PRINCIPLE trial results further confirm prior evidence supporting the use of budesonide in patients with COVID-19 infection.
Important Notice
*medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.