The global public health emergency wrought by the coronavirus disease 2019 (COVID-19) pandemic can be mitigated with safe, effective, and targeted preventive and therapeutic strategies. While vaccination campaigns have been rolling out at an impressive speed in many parts of the world, the lack of an effective anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) drug and the emergence of mutated variants are raising concerns globally. Thus, novel compounds with effective antiviral activity are an urgent requirement for the treatment of severe COVID-19.
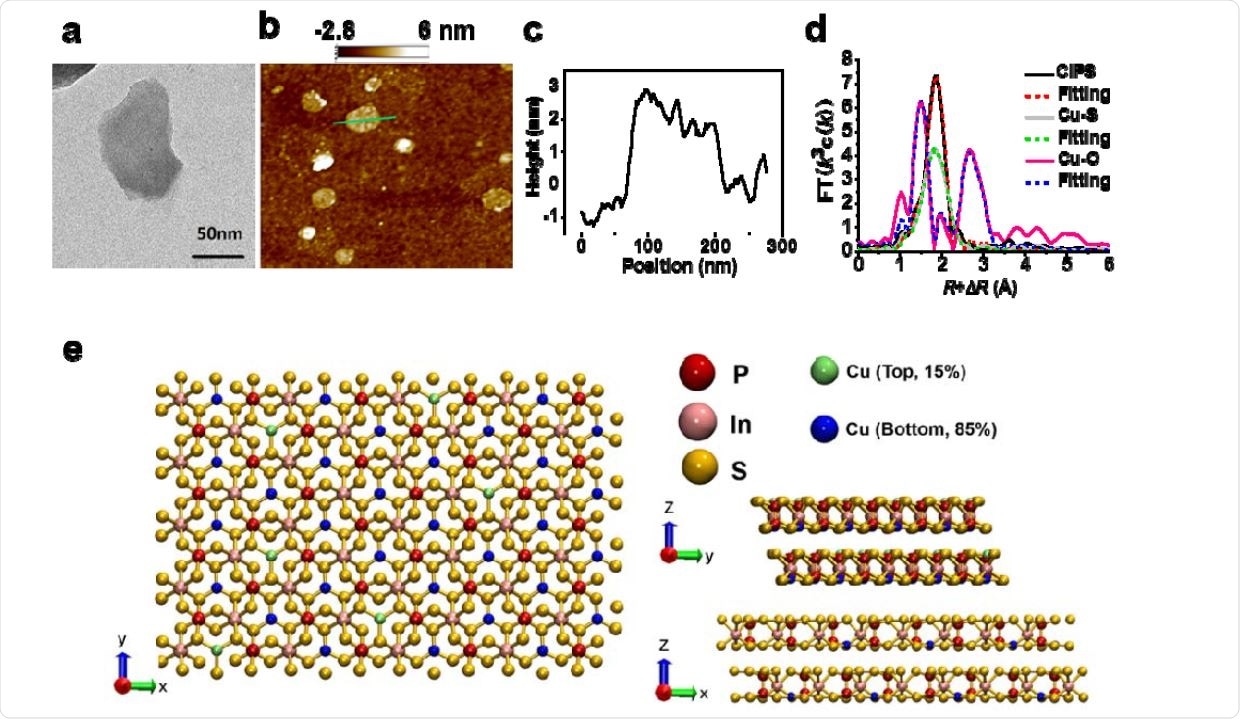
Characterization of CIPS NS. a) Representative TEM image of exfoliated CIPS NS. b-c) AFM image of CIPS NS showing thickness and size distribution. d) Coordination structure of Cu with S atoms in CIPS as determined by EXAFS. e) Schematic illustration of the CIPS crystal structure from top, left and right sides.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The researchers found that this high and selective binding capacity (KD < 1 pM) of CIPS nanosheets with the RBD of SARS-CoV-2 spike protein inhibits viral infection in ACE2-bearing cells and human airway epithelial organoids. They observed that the CIPS displays nano-viscous properties – hence the name ‘nano glue.’ They also found that it has negligible toxicity both in vitro and in vivo.
The researchers pointed out that the antimicrobial and antiviral capacity of nanomaterials (NMs) with their tunable properties has raised particular interest given novel anti-SARS-CoV-2 strategies. They discovered that the copper indium thiophosphate (CuInP2S6, CIPS) nanosheets (NS), an ultrathin 2D and ferroelectric nanomaterial exhibits powerful anti-SARS-CoV-2 capacity.
It is well established that the RBD of the spike protein binds to the angiotensin-converting enzyme 2 (ACE2) on the human host cell, leading to the viral entry. In the paper, the researchers noted that according to Adaptive Poisson-Boltzmann Solver (APBS) electrostatics calculations and the crystal structure of the RBD and ACE2 interface, the surface for RBD is composed of abundant β-sheet structure, which serves as a target for efficient drug design. The computational simulations, consistent with the experimental study, showed that CIPS/RBD exhibits the lowest interaction energy and the strongest electrostatic attraction.
Because of the structural similarity of β-sheet, the RBD preferentially adsorbs on the two-dimensional (2D) NM surfaces with abundant negative charges. This allows for screening a series of NMs for antiviral activity. The CIPS nanosheets used in this study firmly adsorbs the S protein RBD.
This study demonstrated that the CIPS efficiently inhibits the infection of the SARS-CoV-2 virus and explored the mechanism involved. This inhibition of SARS-CoV-2 infection of host cells is possible due to the following: 1) the physical nature of the binding; 2) the chemical component (Cu and S); 3) the electronic structure (with plenty of sulfur atoms, high average surface atomic and charge density, as well as the easy drifting of electrons for copper atoms in the crystal); and 4) the further induced RBD conformational change.
The team also noted that the CIPS nanosheets promote phagocytosis. As macrophages are responsible for eliminating the NMs, the researchers observed that the SARS-CoV-2 trapped by CIPS nanosheets was largely phagocytosed by macrophages, thereby promoting viral elimination.
Importantly, this study highlights that the CIPS has a high affinity with mutant N501Y RBD of SARS-CoV-2’s S protein. Based on the efficacy and excellent biocompatibility of CIPS nanosheets, the researchers recommend it as a promising anti-SARS-CoV-2 drug.
The viscous flypaper-like and selective binding capacity of CIPS for the SARS-CoV-2 S protein also makes it particularly promising as surface coating material and disinfection agents to contain the SARS-CoV-2 spreading.”
The data observed in this study suggests that the selective and preferable binding to the S protein RBD makes CIPS a potentially effective neutralizing drug for COVID-19 treatment. Notably, the CIPS binding is 100x stronger than that described for the best nAbs
To develop a promising new tool to prevent/treat COVID-19, the researchers here proposed to develop an S protein-targeting viscous nanodrug that could combine high antiviral efficacy with excellent biocompatibility, easy preparation, and convenient storage characteristics.
The CIPS nanosheets could thus be a promising nanodrug for future safe and effective anti-SARS-CoV-2 therapy, as well as for use as disinfection agent and surface coating material to constrain SARS-CoV-2 from spreading.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zhang, Guofang et al. (2021). Spike Protein Targeting "Nano-Glue" that Captures and Promotes SARS-CoV-2 Elimination. bioRxiv 2021.04.13.439641; doi: https://doi.org/10.1101/2021.04.13.439641, https://www.biorxiv.org/content/10.1101/2021.04.13.439641v1
- Peer reviewed and published scientific report.
Zhang, Guofang, Yalin Cong, Feng-Liang Liu, Jiufeng Sun, Jiantian Zhang, Guoli Cao, Lingqiang Zhou, et al. 2022. “A Nanomaterial Targeting the Spike Protein Captures SARS-CoV-2 Variants and Promotes Viral Elimination.” Nature Nanotechnology, August, 1–11. https://doi.org/10.1038/s41565-022-01177-2. https://www.nature.com/articles/s41565-022-01177-2.