Researchers in the United States have conducted a study demonstrating the effectiveness of first-generation vaccines at protecting against variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals who have previously been infected with the virus.
The novel SARS-CoV-2 virus is the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic that continues to pose a threat to global public health and has now claimed the lives of more than 3.19 million people worldwide.
The team assessed levels of neutralizing antibodies against variants of SARS-CoV-2 before and after two doses of the Pfizer-BioNTech vaccine in ten individuals who had been infected with the original strain of the virus prior to vaccination. These neutralizing titers were compared with those of people who also received the two vaccines but had not previously been infected.
The researchers, from Oregon Health & Science University in Portland, found that vaccination boosted pre-existing levels of antibodies against the viral spike protein 10-fold in the previously infected individuals, but not to levels that were significantly higher than those of previously uninfected vaccinees.
The spike protein is the main structure the virus uses to bind to and infect host cells. This spike binds to the human host cell receptor angiotensin-converting enzyme 2 (ACE2) via its receptor-binding domain (RBD), a primary target of neutralizing antibodies following natural infection or vaccination.
However, vaccination increased neutralizing antibody titers to higher levels in previously infected individuals versus uninfected vaccinees against the original strain of SARS-CoV-2 and three variants of the virus.
Neutralizing antibody titers from the previously infected vaccinees were more than five times higher against the B.1.1.7 variant that emerged in the UK, more than six times higher against the B.1.351 lineage that emerged in South Africa and more than four times higher against the P.1 lineage that emerged in Brazil.
“Our study indicates that a first-generation COVID-19 vaccine provides broad protection from SARS-CoV-2 variants in individuals with previous infection,” writes Fikadu Tafesse and colleagues.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Concerns have arisen regarding the efficacy of vaccines
Since the COVID-19 outbreak first began in late December 2019, researchers have developed several vaccines to protect against SARS-CoV-2 infection that have completed clinical trials and been authorized for emergency use.
After completion of phase 3 trials, the reported efficacies of vaccine candidates from Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), and Janssen (Ad26.COV2.S) were 95%, 94%, and 66%, respectively.
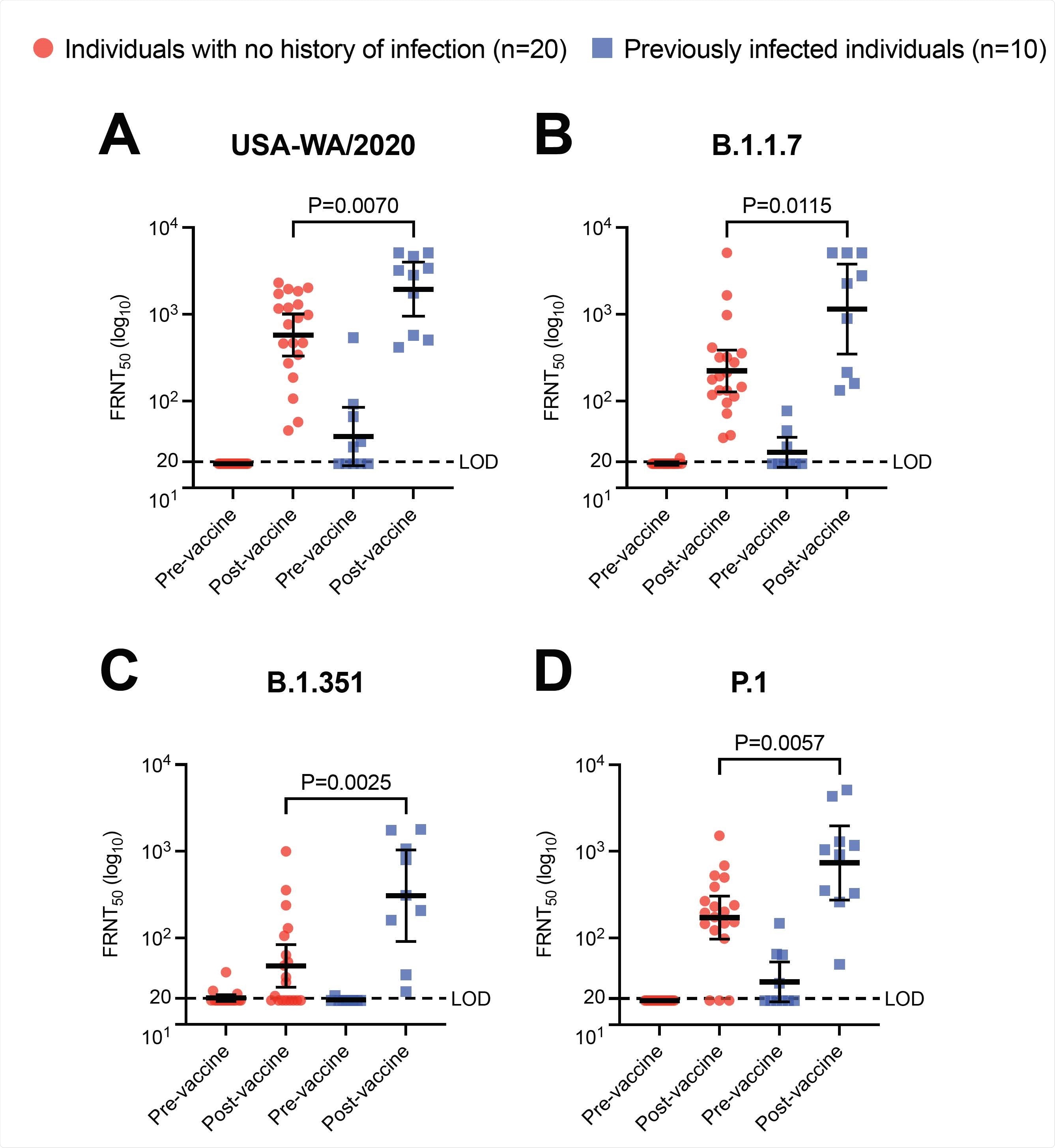
Immunity from natural SARS-CoV-2 infection boosted by subsequent vaccination broadens protection against variants of concern. Neutralization of SARS-CoV-2 variants by pre-and post-vaccination sera collected from previously infected and naïve individuals. Shown are the results of a 50% focus reduction neutralization testing (FRNT50) for an early SARS- CoV-2 isolate USA-WA1/2020 (A) and VOC clinical isolates of B.1.1.7 (B), B.1.351 (C), and P.1 (D). Horizontal bars represent geometric mean titer and I bars represent 95% confidence intervals. Statistical comparisons were made using the Wilcoxon rank-sum test. LOD denotes limit of detection.
In combination with the substantial proportion of many communities estimated to have gained natural immunity through SARS-CoV-2 infection, the rollout of vaccination has raised the possibility that high levels of herd immunity could soon be reached.
However, casting doubt on this prospect is the emergence of SARS-CoV-2 variants of concern (VOCs) containing spike mutations that enhance binding of the virus to host cells and, therefore, transmissibility.
“More concerning has been the emergence of spike mutations with the potential to escape neutralizing antibodies raised against earlier lineages of SARS-CoV-2 through infection with an original lineage or by first-generation COVID-19 vaccines,” writes Tafesse and colleagues.
Recent studies have indeed demonstrated surges of reinfections in regions with the extensive transmission of the South African B.1.351 and Brazilian P.1 lineages, as well as declines in vaccine efficacy against B.1.351.
What did the current study find?
Now, Tafesse and the team have shown that while two doses of the Pfizer BioNTech BNT162b2 vaccine boosted anti-spike antibody titers ten-fold in ten previously infected individuals, these titers were not significantly higher than those elicited in twenty age- and sex-matched previously uninfected vaccinees.
However, in the previously infected individuals, vaccination generated significantly higher levels of neutralizing activity against every SARS-CoV-2 lineage tested than in the previously uninfected vaccinees. Neutralizing antibody titers were 3.5 times higher against the original lineage, 5.2 times higher against B.1.1.7, 6.5 times higher against B.1.351 and 4.3 times higher against P.1.
Importantly, there was no significant difference between the post-vaccination neutralizing antibody titers against the B.1.351 variant in previously infected individuals and those against the original viral strain in previously uninfected individuals.
The team says this finding suggests that first-generation COVID-19 vaccines could retain almost complete efficacy against even the most resistant VoCs when administered following natural infection.
What did the authors conclude?
“Overall, our findings provide important evidence for broad and potent neutralizing antibody responses against emerging SARS-CoV-2 variants, even with exposure to only wildtype SARS- CoV-2 antigen,” writes Tafesse and colleagues.
The researchers say the findings support a recent report that natural infection with B.1.351 induces a similar cross-reactive neutralizing antibody response against B.1.351, P.1, and the original SARS-CoV-2.
“While these and other laboratory results must be validated by ongoing population-level studies, they indicate a novel role for COVID-19 vaccines in protecting hard-hit populations from future waves of the pandemic,” concludes the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.