A vexing problem in the development of vaccines has been the need to achieve the right glycosylation profile for the viral antigens. A new study, recently released as a preprint on the bioRxiv* server, shows how a sequential cell-free approach produced complex N-glycans from simpler types, in a one-pot reaction.
This could be used to rapidly and easily obtain any of a large array of N-glycans on potential vaccine antigen candidates in order to carry out animal trials and preclinical testing, such that it is possible to lay out their impact on the immune response and enhance the efficacy of protein subunit vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Protein glycosylation
Glycosylation of proteins is critical in determining many biological characteristics of a protein, including its structure, function, solubility, trafficking within and through cell membranes and binding to ligands. The distribution and excretion of the compound, as well as its conversion to other metabolites, are also dependent to a large extent on the addition of glycosyl residues.
When it comes to viral particles, glycosylation affects viral attachment to the host cells as well as the release of decoy particles to sidetrack immune antibodies and cells. Glycosylation is also key to immune-evading epitope shielding, using the cell’s own glycosylation apparatus.
This is a major hurdle for vaccine development. Moreover, not just the presence of N-glycans but the glycoform itself affects the binding of the virus, its infectiousness, antigenicity and immunogenicity. The resemblance achieved between viral immunodominant antigens and host cell proteins by their surface glycosylation profile is thought to benefit the virus.
On the other side, specific glycosyl residues could be manipulated to improve vaccine efficacy, argue the researchers. This remains an area of open and ongoing research.
The SARS-CoV-2 spike glycosylation profile
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a virus with single-stranded ribonucleic acid (RNA). It has three immunogenic membrane proteins, the spike or S protein, an integral membrane protein and an envelope protein, which comprise its envelope. The S glycoprotein engages the host cell angiotensin-converting enzyme 2 (ACE2) receptor to accomplish viral entry.
The S protein is heavily glycosylated, with over a score of N-glycosylation sites, more than many other viruses.
In vitro glycoengineering
These residues are often studied in cell lines such as the HEK cells, where unique glycosylation profiles are produced on the proteins. This requires specific protocols to be used with each distinct cell line, making it labor-heavy.
Glycoproteins vary between themselves and even within the same class, making it an additional challenge to understand how exactly they shape the immune response in animal models.
As an efficient alternative, techniques for the production of both eukaryotic and bacterial glycosyltransferases within one-pot cell-free settings have been carefully honed to allow glycan processing. This can be used to engineer glycoforms on proteins, irrespective of the cell system used for protein expression.
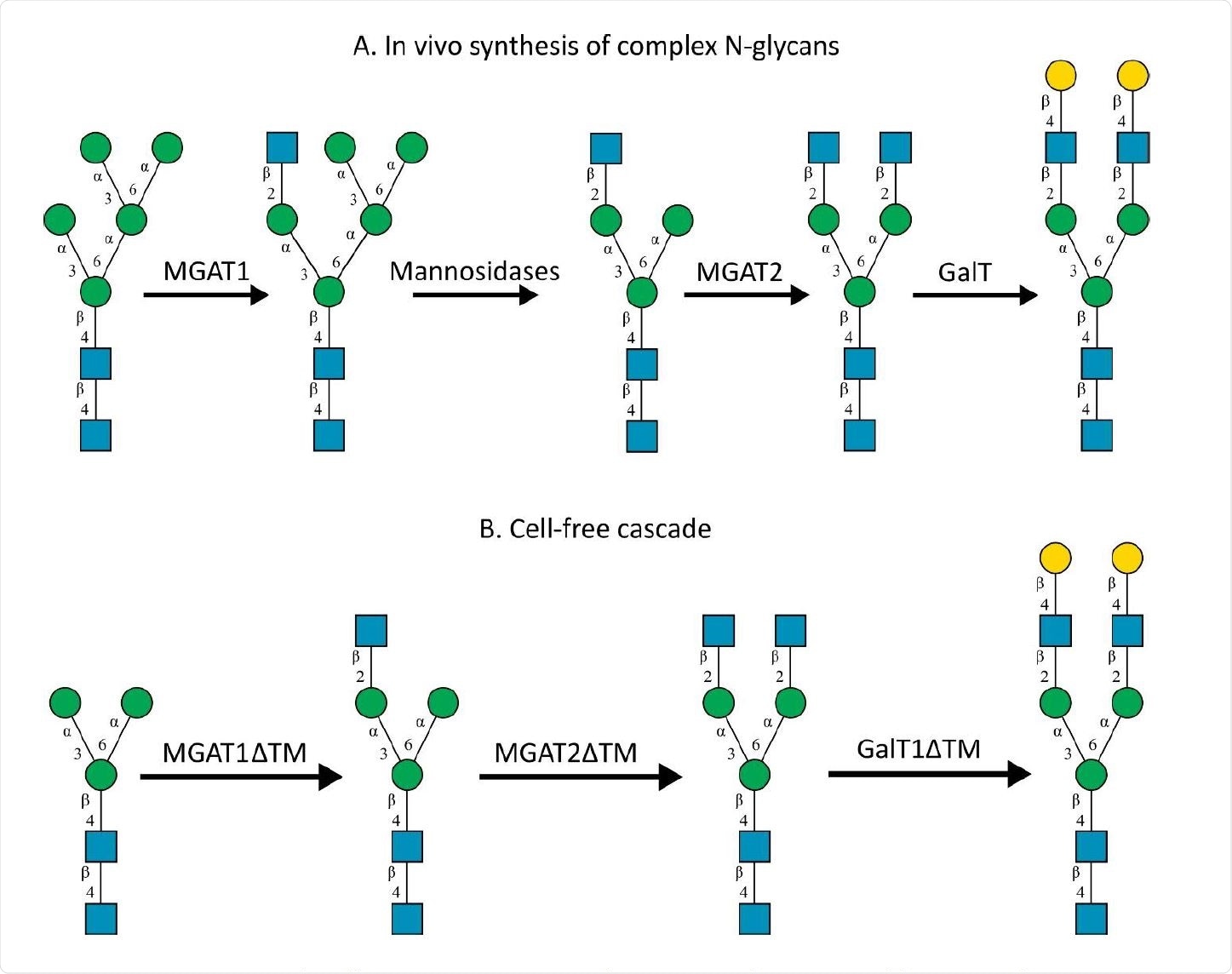
A) In-vivo the oligomannose-type N-glycan Man5 is converted into complex-type glycans by mannosidases and MGAT1, MGAT2 and GalT. Substrates for these reactions are UDP-GlcNAc and UDP-galactose, respectively. B) This process can be remodelled in-vitro to synthesize complex-type structures on insect cell-derived recombinant proteins with paucimannose-type N-glycans, like Man3.
What are the results?
In the current study, a cell-free sequential one-pot reaction was utilized to successfully convert fucosylated and afucosylated paucimannose-type N-glycans to complex-type galactosylated N-glycans.
The current study combined recombinant human glycosyltransferases in a cell-free one-pot reaction, since they are known to be expressed in E. coli and to effectively glycosylate the recombinant proteins in this setting.
The study used three glycosyltransferases co-expressed in E. coli to convert the typical simple, low-mannose, N-glycans of the recombinant SARS-CoV-2 spike into the classic mammalian complex galactosyl-rich structures.
The findings indicate that mannose-3-glycans, both with and without fucosylation, were largely converted to the corresponding galactosylated residues.
Eight of 22 N-glycosylation sites were rich in oligomannose-type N-glycans, probably more because of steric hindrance to glycan processing enzymes in the processing areas, rather than because of the cell line used.
The engineered spike protein in this setting had abundant complex galactosylated N-glycans, with some hybrid and oligomannose-type N-glycans as well. However, complex-type N-glycans on human cell-derived spike proteins also show sialic acid residues and multi-antennary structures.
Applications of in vitro glycoengineering
The insect cell expression system they used with baculovirus inoculation is capable of efficient processing of eukaryotic protein, is easily scalable and turns out large amounts of engineered protein, making it ideal for protein subunit vaccine manufacture.
The glycoform produced in such systems is quite distinct from that produced in mammalian cells, in that insect cells express mostly low-mannose hybrid N-glycans, but the latter, complex oligomannose-type N-glycans. However, the pure spike variants produced in the current study showed the latter pattern, which is very unusual for this expression system.
Three vaccines produced by this pathway have already been licensed, namely, Flublok®, Cervarix® and Provenge®, against influenza, human papillomavirus, and prostate cancer. Many more, including some against COVID-19, are being developed.
A recombinant spike ectodomain produced by an insect cell expression system has already been shown to be very immunogenic in non-human primate studies. Using this technology, one protein subunit vaccine (RBD-Dimer) against COVID-19 is currently approved in China. The Novavax and Glaxo-Smith-Kline vaccines are awaiting approval.
The use of non-human glycans that incorporate N-glycolylneuraminic acid or α,1-3-linked galactose residues could increase the efficiency of immune responses, but more work is required to rule out their allergenicity.
Taken together, a significant overlap of the glycoform has been generated. Whether the overlap is also site-specific remains to be investigated in future.”
What are the implications?
The in vitro glycoengineering approach to produce complex-type N-glycans by modifying insect cell lines offer the independence of producer cell line, and increased versatility to allow many different glycoforms if they are structurally similar.
The availability of sugar nucleotides, which are the substrates for this process, at commercial rates will be key to upscaling this technology.
The use of transient insect cell lines, is one such in vitro glycoengineering approach, but its stability in large-scale production has not been confirmed. On the other hand, the simultaneous expression of glycosyltransferases to produce complex-type N-glycans, as used by the current researchers, places more demands on the cell metabolism and reduces cell growth.
While much more work remains to be done, in vitro glycoengineering may be of great use to produce potential vaccine antigens and to understand how glycosylation affects the immune response. Overall, these strategies could be used to produce highly customized glycoforms for leading viral vaccine candidates, including platforms like activated and attenuated viruses, or virus-like particles.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ruhnau, J. et al. (2021). Cell-free glycoengineering of the recombinant SARS-CoV-2 spike glycoprotein. doi: https://doi.org/10.1101/2021.04.30.442139, https://www.biorxiv.org/content/10.1101/2021.04.30.442139v1
- Peer reviewed and published scientific report.
Ruhnau, Johannes, Valerian Grote, Mariana Juarez-Osorio, Dunja Bruder, Reza Mahour, Erdmann Rapp, Thomas F. T. Rexer, and Udo Reichl. 2021. “Cell-Free Glycoengineering of the Recombinant SARS-CoV-2 Spike Glycoprotein.” Frontiers in Bioengineering and Biotechnology 9 (August). https://doi.org/10.3389/fbioe.2021.699025. https://www.frontiersin.org/articles/10.3389/fbioe.2021.699025/full.