As over a dozen vaccines are being rolled out worldwide, to protect against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and many more are still being developed, it is necessary to examine the immune response and to understand how and to what extent these vaccines are protective against the virus.
The rapid pace of vaccination in many developed countries has succeeded in driving down the rates of infection and of death in such regions.
A new study released as a bioRxiv* preprint tried to establish how tissues such as the upper respiratory tract (URT) – which harbor the virus and often act as the point of entry – are difficult to access for routine testing, but are responsive to vaccination.
The study focused on identifying characteristics of the adaptive immune T cells in the nasal mucosa, before and after vaccination with the Pfizer-BioNTech mRNA vaccine.
Cell-mediated immunity is key in long-term protection against SARS-CoV-2 infection, as well as neutralizing antibodies. Such cellular immunity continues to be detectable in the blood for eight months, at least, following natural infection.
In non-human primate studies, the depletion of CD8+ T cells resulted in higher chances of reinfection.
Study details
The current study, therefore, uses a cytometry-based assay to categorize immune cells from nasopharyngeal (NP) swabs obtained for COVID-19 testing among healthcare workers.
Interestingly, CD45+ T cells were recovered from samples collected with one type of swab (Flexible minitip flocked swab, BD), but CD326+ cells from two other types of swabs. This shows that certain types of NP swabs are more useful in retrieving nasal T cells.
Moreover, a pilot study of eight samples showed that the BD swab yielded over 3,000 CD45+ cells per swab, of which most were CD3+ cells. However, almost all CD8+ T cells carried CD69 and CD103 markers, indicating their identity as tissue-resident memory (Trm) cells, while this was the case with 10-80% of CD4+ T cells.
The study included 21 volunteers who gave pre- and post-vaccine samples, the latter being collected at 12 days after the first and second doses. The median age was 40 years, and two out of three were female. Most had taken their flu shots in the last few months, and two had a history of COVID-19 infection before the scheduled vaccination.
What were the findings?
The researchers found that Trm CD8+ T cells increased significantly at both post-vaccine points, by 0.31 and 0.44 log10 cells per nasal swab, respectively. However, this was not observed in CD4+ Trm cells.
In terms of absolute numbers, here, too, the Trm CD8+ T cells increased by 0.06 log10 cells/swab after the first dose, and by 0.48 log10 cells/swab after the second dose. Moreover, this was accompanied by a corresponding decrease in the already low numbers of these cells in blood, indicating that a migration occurred into the tissues.
CD4+ T cells bearing the Th17 markers CD161 and CCR6 increased by 0.40 and 0.45 log10 cells/nasal swab post-vaccination, but nasal T follicular helper (Tfh) cells were not increased.
The observed changes were similar in both sexes and in those with prior infection.
Earlier studies using other vaccines have shown evidence of T cell activation, in the form of markers like Ki67, HLA-DR, and CD38. Using these, vaccine-induced T cells were identified at 10-14 days from vaccination. In the current study, however, these were not seen in either CD4+ or CD8+ T cells, in blood or the nasal mucosa.
Overall, there was an increase in the abundance of cells expressing CD45+, CD3+, and CD8+ after the second dose of the vaccine. Thus, it seems that several immune cell subsets increase in number after vaccination.
The study also indicates an increase in activated cytotoxic T cells following vaccination. When stimulated by pooled SARS-CoV-2 spike peptides, NP swab cells taken from vaccinated individuals at two months from the second dose expressed higher levels of the cytotoxic marker, CD107a and activation marker CD154. These were also seen with both TNF-α and perforin.
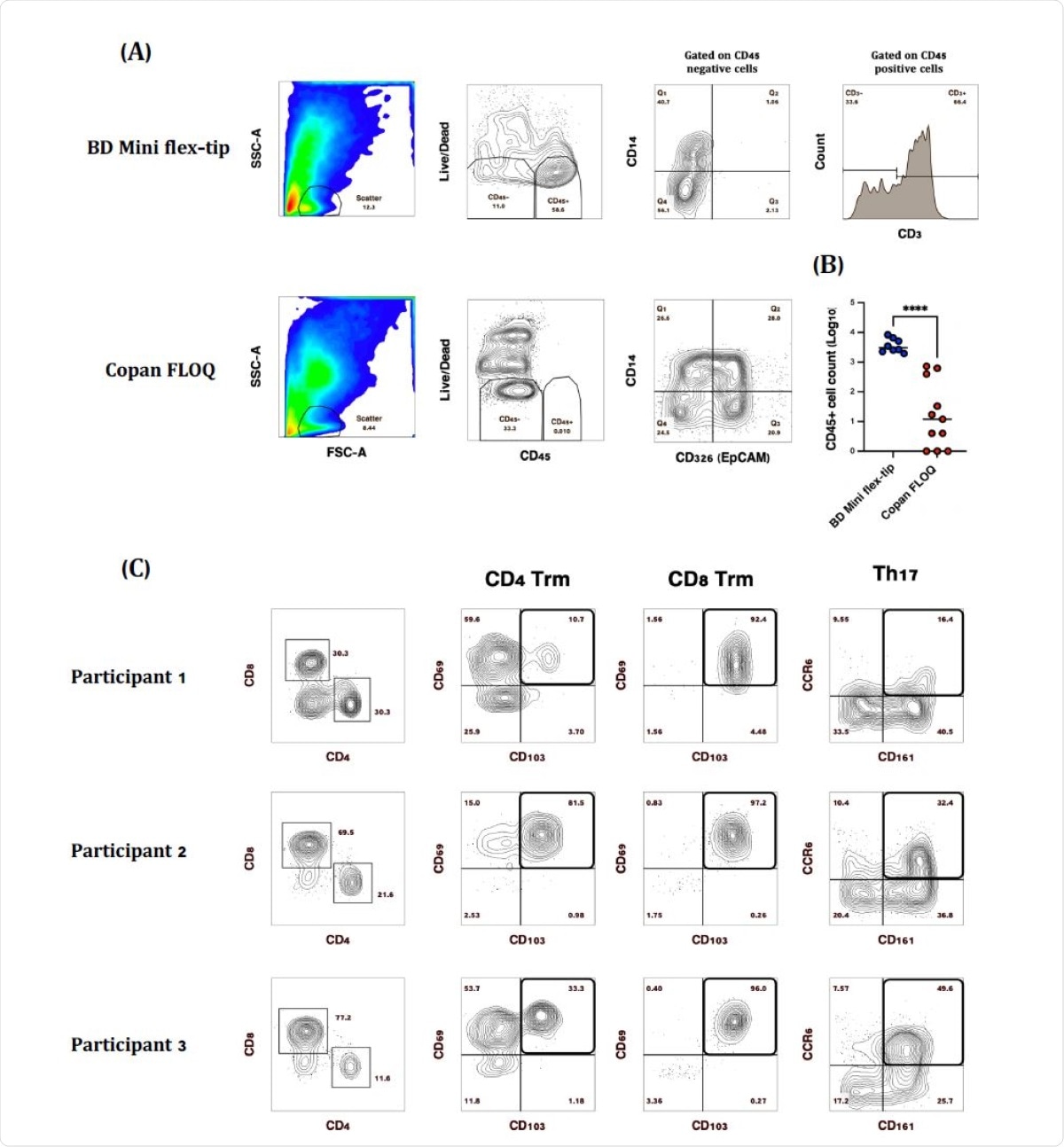
Comparison of SARS-CoV-2 diagnostic testing nasopharyngeal (NP) swabs for immune cell recovery. Representative plots of CD45+ nasal immune cells isolated from (A) BD Mini Flex-tip swabs, majority of which (~40-90%) were CD3+ and Copan FLOQ swabs from which epithelial (CD326 (EpCAM+) cells were the main cell type recovered. (B) Swab brand comparison of CD45+ immune cell recovery. (C) Representative plots of CD3+ T cell subsets isolated from BD Mini-tip flexible swabs. Majority of CD4+ and CD8+ T cells were Trm based on the expression of CD69 and CD103, while 16-50% of CD4 T cells were Th17 based on the expression of CD161 and CCR6. (****P>0.0001)
Of course, Immunoglobulin (Ig) G antibody titers increased after the first dose, and still more markedly after the second dose. Only one of the two individuals with prior infection showed anti-nucleocapsid IgG. However, there was no correlation between the increase in spike-specific antibodies and the increase in CD8+ Trm and CD4+ Th17 cells in the nasal mucosa, which were similar in all participants.
These data suggest that tissue T cell responses to vaccination may be independent of the antibody response and may provide an additional layer of immunity against SARS-CoV-2 infection.”
What are the implications?
Trm cells patrol the body’s internal surfaces in order to rapidly trigger immune responses to pathogens that have been encountered and overcome before. When activated, they lead to the overexpression of homing markers, surface molecules that direct their passage to specific organs where a high fraction of them are retained over the long term to act as sentinels against reinfection.
This location has made it hard to study them. However, the current study indicates the possibility that some commonly used NP swabs are effective in obtaining such cells from nasal tissue.
The increase by half an order of magnitude in many subsets of both CD4+ and CD8+ T cells in nasal tissue after vaccination with the Pfizer vaccine is an important finding. This observation confirms mouse studies showing that nasal Th17 Trm cells are elicited by parenteral vaccines and protect against the establishment of bacterial colonies. Z
The current study is the first human study to show the effect of injectable vaccines against COVID-19 on nasal tissue-resident memory T cells. These cells may be induced by vaccination to add to the protective immunity against the virus.
Earlier studies have shown that antibodies and lung T cells increase following viral challenge. Lung cells do inhabit tissue, but may not offer the primary defense against SARS-CoV-2. Instead, some scientists think that Trm are, indeed, “the most effective form of T cell response against SARS-CoV-2 infection, capable of preventing viral dissemination beyond the URT [upper respiratory tract], where more virus-induced damage can occur.” This aspect deserves further exploration to increase the efficacy of protection against this infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources