Preliminary clinical trial results from an ongoing Phase 2/3 trial led by Laurent M. Humeau of Inovio Pharmaceuticals suggest the two-dose INO-4800 vaccine is safe for use in adults of varying ages. The results expand on phase 1 trial results that found the vaccine was safe and tolerable in 38 participants.
INO-4800 is a plasmid DNA vaccine delivered by electroporation and contains the S1 and S2 subunits of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein.
The findings suggest INO-4800 could be a potential vaccine candidate. Future work would focus on a phase 3 trial investigating the vaccine's effectiveness in protecting people against SARS-CoV-2.
The research "Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure" is available as a preprint on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Clinical trial design
The researchers designed a multicenter clinical trial testing the immunogenicity and efficacy of the two-dose DNA vaccine when delivered via electroporation. They also evaluated how safe and effective the vaccine was when given at 1.0 mg versus 2.0 mg.
Eligible participants included healthy, seronegative adults working in an environment where the risk of exposure to SARS-CoV-2 was high. Participants also had to have medically effective contraception, were postmenopausal, surgically sterile, or have a sterile partner.
A total of 401 participants enrolled in the study from November 30, 2020, and February 5, 2021.
201 participants were randomly assigned either one or two injections of the INO-4800 vaccine at a dose of 1.0 mg or one or two placebo injections. The first dose would be delivered on the first day of the trial. The second dose was injected on day 28.
Injections were given over the deltoid or anterolateral quadricep muscles, followed by electroporation. The second vaccine was injected in a different arm or leg from the first dose.
200 participants were randomly assigned two vaccine doses at 2.0 mg or a placebo in two different limbs.
The researchers measured for any immediate or delayed side-effects after the first dose, on day 7, on day 28 after the second dose, on day 45, day 56, day 210, and day 392. Participants were encouraged to record any local or systemic adverse events in a diary. Blood work and urine analysis were also performed on the first day, day 28, day 42, and day 392 after the first dose.
Participants that developed symptoms related to COVID-19 underwent diagnostic testing. If they had COVID-19 before receiving the second dose, they were not allowed to get it.
Cellular and humoral samples were collected before administering the first dose on day 0, day 42, day 210, and day 392.
Vaccine safety and reported adverse events
There were 1,679 adverse events reported in 300 participants after week 8, with 1,446 treatment-related adverse events in 281 participants. The most common adverse events observed in 5% of participants included injection site reactions, fatigue, headache, muscle pain, and nausea.
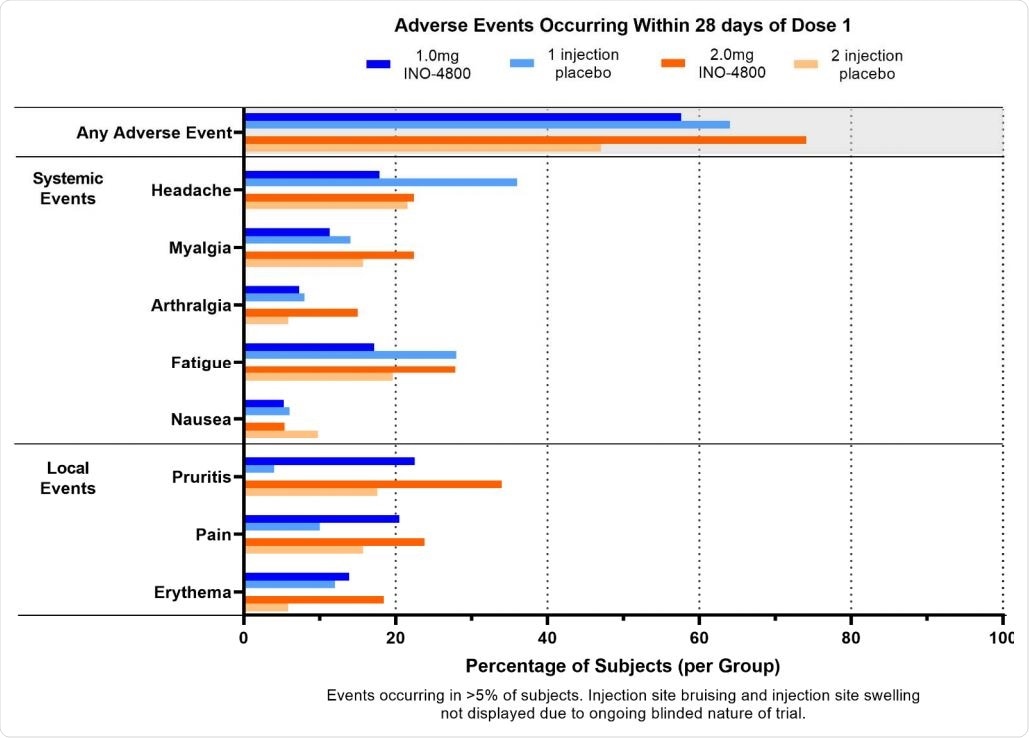
Adverse Events occurring within 28 days of dose 1
Most adverse events were of Grade 1 and Grade 2 severity. These adverse events did not further increase after the second dose. There were three Grade 3 adverse events, including muscle pain but reported cervical dysplasia and skin laceration were not treatment-related.
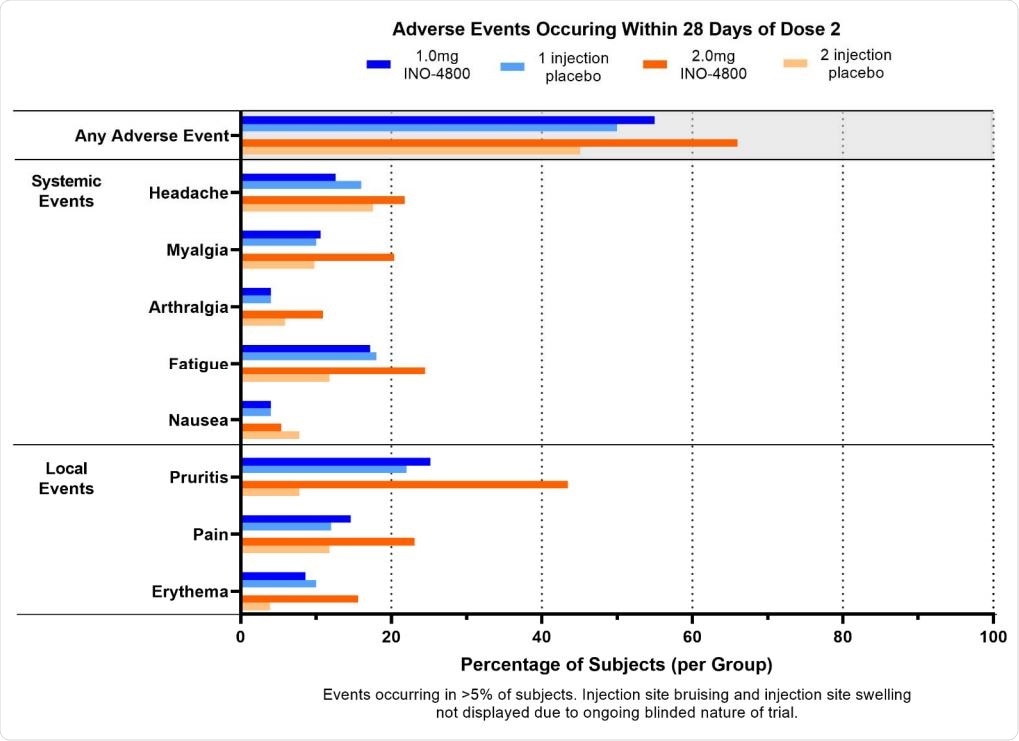
Adverse Events occurring within 28 days of dose 2
Vaccine-induced immune response
After week 6, both the 1.0 mg and 2.0 mg dose groups showed humoral immune responses. Although, there was a greater humoral immune response associated with the higher dose.
When testing the participants' serum, the researchers found both dose groups produced neutralizing antibody responses specific to SARS-CoV-2. Again, the 2.0 mg dose group had statistically significantly higher antibody responses than the 1.0 mg dose group.
Antibody responses differed amongst age groups.
The researchers also observed an increase in T cell immune responses after week 6. The magnitude of IFN trended higher amongst the 1.0 mg vaccine group compared to the 1-injection placebo group. Participants given the 2.0 mg vaccine dose had higher T cell responses than the 2-injection placebo groups.
Based on the results, the researchers suggest the results further support the potential for INO-4800 in protecting against SARS-CoV-2. A planned Phase 3 trial can further confirm these results.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mammen MP, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv, 2021. doi: https://doi.org/10.1101/2021.05.07.21256652, https://www.medrxiv.org/content/10.1101/2021.05.07.21256652v1
- Peer reviewed and published scientific report.
Kraynyak, Kimberly A, Elliott Blackwood, Joseph Agnes, Pablo Tebas, Mary Giffear, Dinah Amante, Emma L Reuschel, et al. 2022. “SARS-CoV-2 DNA Vaccine INO-4800 Induces Durable Immune Responses Capable of Being Boosted in a Phase 1 Open-Label Trial.” The Journal of Infectious Diseases 225 (11): 1923–32. https://doi.org/10.1093/infdis/jiac016. https://academic.oup.com/jid/article/225/11/1923/6515374.