A team of scientists from the USA and Belgium has recently revealed that the ErbB family of receptor tyrosine kinases play a vital role in regulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lifecycle in host cells and that inhibition of these receptors can protect against SARS-CoV-2-induced acute and chronic lung injuries and inflammation. The study is currently available on the bioRxiv* preprint server.
Since the beginning of the coronavirus disease (COVID-19) pandemic, the development of therapeutic and preventive measures against SARS-CoV-2 has been the primary area of focus in global research. Many potent vaccines have been developed at record speed, in addition to repurposing already approved antiviral medicines to tackle SARS-CoV-2 transmission.
Currently, the standard of care treatments for in-hospital severe COVID-19 patients include corticosteroids, interleukin receptor antagonists, and antiviral medicine remdesivir. Although capable of reducing symptom severity and disease progression, none of these medicines have shown life-supporting efficiency in these patients.
In the current study, the scientists have conducted a high-throughput screening to identify compounds that can protect mammalian cells from SARS-CoV-2-induced lethality.
Screening of compounds
The scientists screened a total of 4,413 bioactive compounds and FDA-approved drugs to identify potent anti-SARS-CoV-2 compounds. In the high-throughput screening, the ability of these compounds in inhibiting SARS-CoV-2-induced cellular lethality was investigated.
A total of 18 candidates were identified and tested for their efficacy in preventing SARS-CoV-2 infection in a dose-dependent manner. Of 18 compounds, 17 showed potential antiviral effects without causing toxicity. Of these compounds, 12 were effective at low micromolar concentrations. The final panel of compounds includes inhibitors of ErbB receptor tyrosine kinases, NUMB-associated kinases, heat shock protein 90 (HSP90), and cell membrane ion transporters.
Antiviral activities of select compounds
The majority of identified compounds showed potent antiviral efficacy against different RNA viruses in a dose-dependent manner. Based on the antiviral efficacy, the scientists selected an already approved pan-ErbB inhibitor, lapatinib, for further experiments.
In in vitro assays, lapatinib dose-dependently inhibited SARS-CoV-2 replication in host cells after 24 hours of infection. In addition, it protected the cells from SARS-CoV-2-induced lethality after 96 hours of infection. Importantly, viruses treated with lapatinib even for a prolonged period of time failed to develop drug resistance.
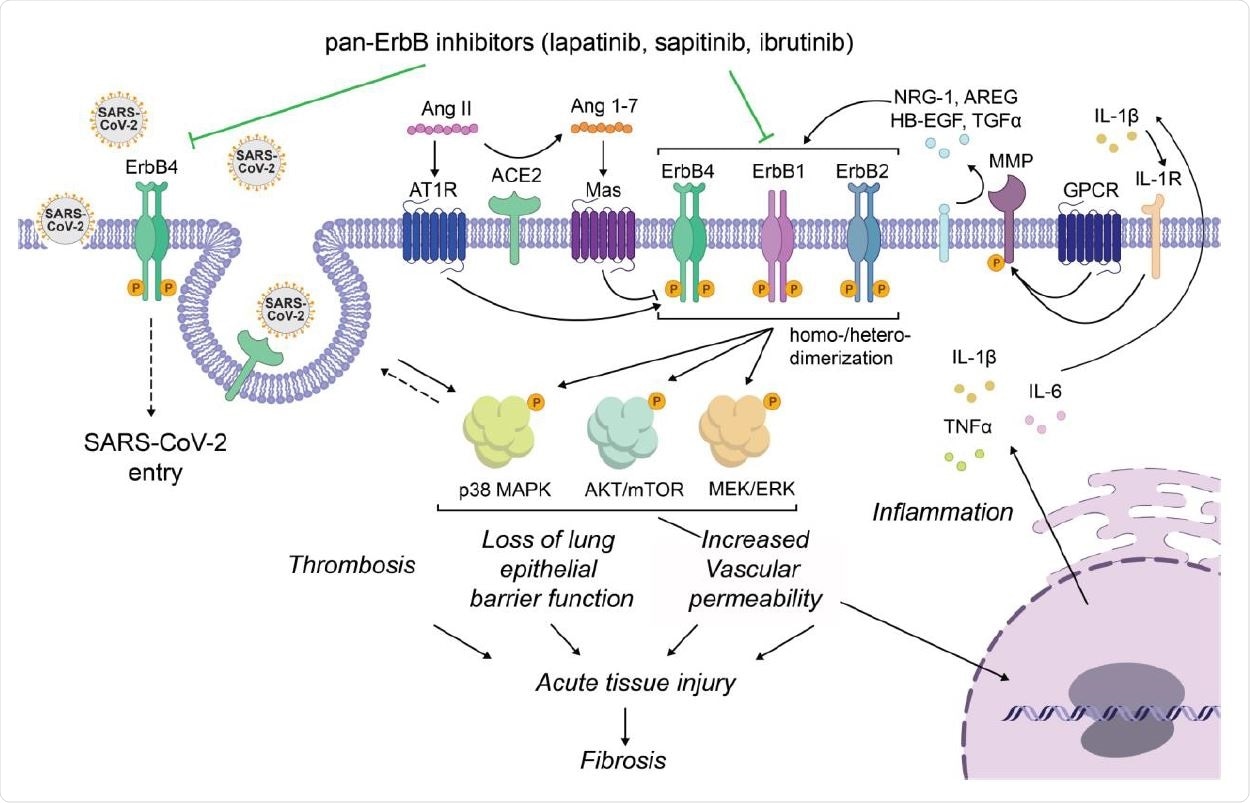
Proposed model for the roles of ErbBs in the regulation of SARS-CoV-2 infection and pathogenesis and the mechanism of action of pan-ErbB inhibitors. By inhibiting ErbB4, lapatinib suppresses SARS-CoV-2 entry. By inhibiting pan-ErbB activation by various ligands and unopposed Ang II effect, lapatinib inhibits activation of signaling pathways known to be activated and deleterious in severe pandemic coronaviral infections, thereby protecting from inflammation and tissue injury.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Antiviral mode of action of lapatinib
While lapatinib inhibited SARS-CoV-2 infection in cells 24 hours after infection, it suppressed intracellular viral RNA levels already at 3 hours after infection. These findings indicate that lapatinib inhibits SARS-CoV-2 infection at the entry stage.
There are three catalytically active members of the ErbB family: ErbB1, 2 and 4. Among 7 molecular targets of lapatinib, siRNA-mediated depletion of ErbB4 resulted in about 85% reduction in virulent SARS-CoV-2 infection and viral entry. These findings highlight the involvement of ErbB4 signaling in mediating SARS-CoV-2 host cell entry.
Findings obtained from a panel of in vitro assays confirmed that ErbB4, but not the other members of ErbB family, is the primary target of lapatinib and that lapatinib exerts its anti-SARS-CoV-2 activity by inhibiting ErbB4 phosphorylation.
In SARS-CoV-2-infected cells, an increased phosphorylation of downstream components of the ErbB4 signaling pathway was observed. Interestingly, treatment with lapatinib caused a significant reduction in SARS-CoV-2-induced phosphorylation of important molecular targets downstream of the ErbB4 pathway.
In a human adult lung organoid monolayer model, lapatinib treatment significantly suppressed the levels of inflammatory cytokines, which were otherwise induced by SARS-CoV-2 infection. Similarly, claudin 7 staining of SARS-CoV-2-infected, lapatinib-treated human lung organoids revealed that lapatinib is capable of preventing SARS-CoV-2-induced disruption of epithelial barrier integrity.
Study significance
The study observations reveal that the activation of the ErbB4 signaling pathway is required for SARS-CoV-2 host cell entry and infection establishment. Lapatinib, an FDA-approved pan-ErbB inhibitor, exhibits potent anti-SARS-CoV-2 activity by primarily inhibiting ErbB4 phosphorylation and activation.
In both in vitro and ex vivo setups, lapatinib treatment has been shown to suppress SARS-CoV-2-induced inflammation and lung tissue injury.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.