Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the novel virus responsible for the ongoing coronavirus disease (COVID-19) pandemic. COVID-19 can cause critical illness characterized by severe pulmonary and extrapulmonary manifestations. The risk of mortality for patients with severe COVID-19 is high.
Critical COVID-19 is characterized by thrombotic coagulopathy. Increased levels of D-dimer and activation of coagulation are associated with worse clinical outcomes. Fibrin deposition is common in the lung vasculature in chronic COVID-19. Research suggests that COVID-19 pathophysiology may be affected by microvascular thrombosis.
Severe COVID-19 may be associated with increased circulating levels of several biomarkers
Analysis of histologic specimens and measurement of circulating proteins and metabolites have shown severe vascular inflammation and endothelial injury. Thus, a phenotypic switch of endothelial cells to a procoagulant state seems to be a critical disease mechanism. The endothelial dysfunction caused by COVID-19 may be mediated by circulating inflammatory cytokines, neutrophil extracellular traps, autoantibodies, and direct viral infection.
Several studies support the theory that severe COVID-19 may be associated with increased circulating levels of these markers, including procoagulant Von Willebrand factor (VWF), vascular cell adhesion markers (VCAM, ICAM, E-selectin), and plasminogen activator inhibitor (PAI-1). Increased circulating antithrombotic endothelial surface proteins tissue factor pathway inhibitor (TFPI) and thrombomodulin, which are removed from the endothelial surface during inflammation, are also said to be associated with severe COVID-19.
In vitro study to determine the association between severe COVID-19 and procoagulant endothelial dysfunction and Tie2-angiopoietin axis changes
Previous studies demonstrated that the dysregulation of the Tie2-angiopoietin pathway is a central link between inappropriate coagulation and vascular inflammation. The Tie2 antagonist angiopoietin-2 (Angpt-2) is released from activated endothelial cells during inflammation, and it inhibits Tie2, thus promoting a prothrombotic phenotypic shift.
Researchers from the US recently assessed if severe COVID-19 is linked to procoagulant dysfunction of the endothelium and changes in the Tie2-angiopoietin axis. They assessed circulating endothelial markers in a study cohort of 98 patients with mild, moderate, or severe COVID-19. This study is published on the medRxiv* preprint server.
Increased disease severity is associated with increased endothelial dysfunction
The assessment showed profound endothelial dysfunction, which indicates a prothrombotic state. Angpt-2 concentrations increased with an increase in disease severity, and the highest levels of Angpt-2 were linked to the worst survival outcomes.
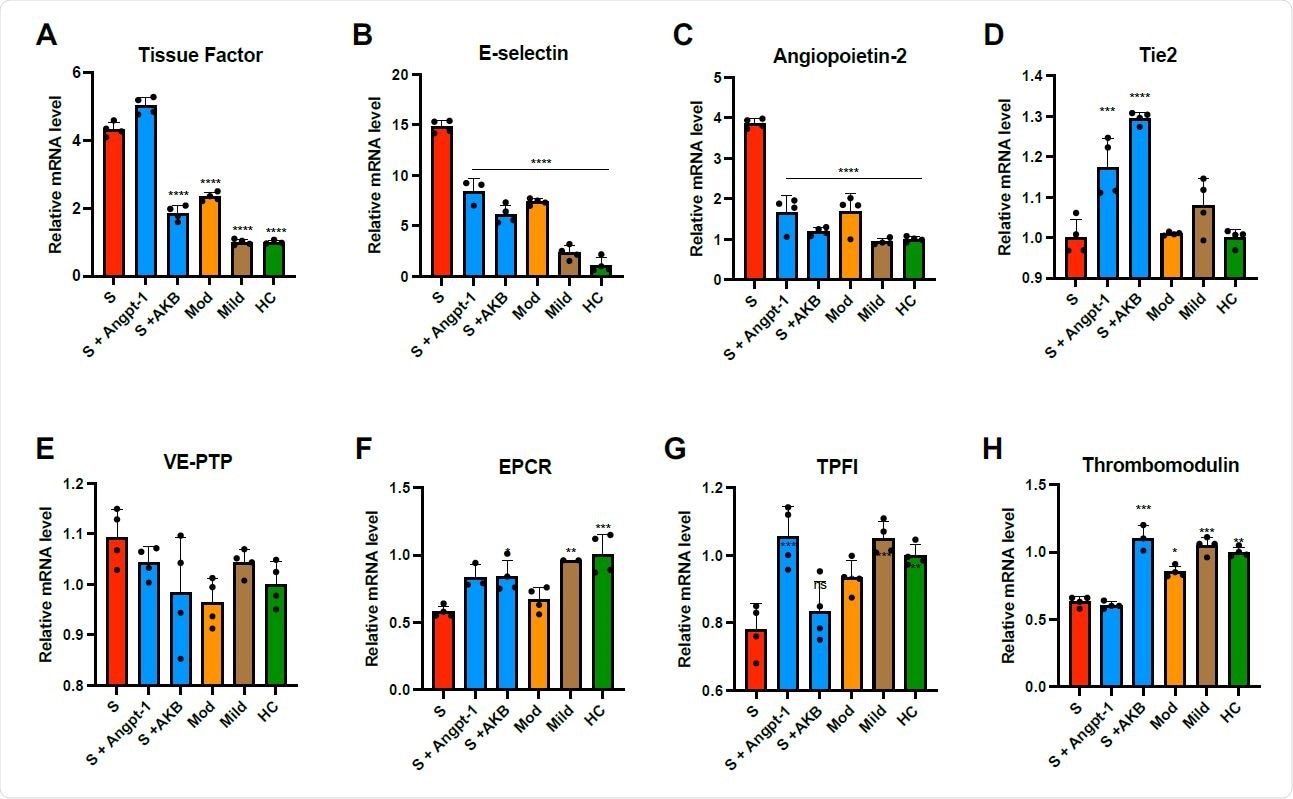
Plasma from patients with COVID-19 induces thromboinflammatory gene expression in endothelial cells. HUVECs were cultured overnight in the presence of 10% pooled plasma from patients with severe (S, ICU patients), moderate (Mod, non-ICU hospitalized patients), or mild (non-hospitalized outpatients) COVID-19 or healthy controls (HC) and analyzed for relative fold mRNA expression change of tissue factor (A), E-selectin (B), angiopoietin-2 (Angpt-2, C), Tie2 (D), vascular endothelial protein tyrosine phosphatase (VEPTP, E) endothelial protein C receptor (EPCR, F), tissue factor pathway inhibitor (TFPI, G), and thrombomodulin (H). When indicated, cells were pretreated with Angpt-1 (300 ng/mL) or AKB- 9778 (5 μM) for 30 min prior to incubation with plasma. Gene expression was normalized to that of actin and changes are shown relative to HC. Graphs represent the mean ± SD. Significance in comparison to severe (S) was determined by 1-way ANOVA using Dunnett post-test, *P < 0.05 **P < 0.01, ***P < 0.001 ****P < 0.0001.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The researchers found that when treated with plasma taken from severe COVID-19 patients, primary human endothelial cells upregulated the expression of thrombo-inflammatory genes, inhibited the expression of antithrombotic genes, and endorsed coagulation on the endothelial surface.
"Our plasma vascular survey demonstrates that increasing severity of COVID-19 is associated with coagulopathy and shows a clear relationship between increasing levels of endothelial cell dysfunction and increasing clinical strata of disease severity."
They also noticed that pharmacologic activation of Tie2 with the AKB-9778 reversed the prothrombotic state caused by COVID-19 plasma in endothelial cells. They examined lung autopsy specimens from severe COVID-19 patients and found a prothrombotic endothelial signature as shown by an increase in von Willebrand Factor and loss of anticoagulant proteins.
Findings support ongoing trials of Tie2 activating therapy with AKB-9778 in severe COVID-19 patients
The authors used an in vitro model to treat primary endothelial cells with COVID-19 plasma to examine the Ang2-Tie2 system in COVID-19-mediate endothelial dysfunction. The study's findings highlight the disruption of Tie2-angiopoietin signaling and procoagulant changes in endothelial cells in severe COVID-19 patients. The data from the study also offer a new rationale for current trials of Tie2 activating therapy with AKB-9778 in patients with severe COVID-19 disease.
"In conclusion, the present study supports the concept that moderate and severe COVID-19 are driven at least in part by procoagulant endothelial cell dysfunction, the degree of which increases in parallel with COVID-19 disease severity."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19, Alec A. Schmaier, Gabriel Pajares Hurtado, Zachary J. Manickas-Hill, Kelsey D. Sack, Siyu M Chen, Victoria Bhambhani, Juweria Quadir, Anjali K. Nath, Ai-ris Y. Collier, Debby Ngo, Dan H. Barouch, Robert E. Gerszten, Xu G. Yu, MGH COVID-19 Collection and Processing Team, Kevin Peters, Robert Flaumenhaft, Samir M. Parikh, medRxiv, 2021.05.13.21257070; doi: https://doi.org/10.1101/2021.05.13.21257070, https://www.medrxiv.org/content/10.1101/2021.05.13.21257070v1
- Peer reviewed and published scientific report.
Schmaier, Alec A., Gabriel M. Pajares Hurtado, Zachary J. Manickas-Hill, Kelsey D. Sack, Siyu M. Chen, Victoria Bhambhani, Juweria Quadir, et al. 2021. “Tie2 Activation Protects against Prothrombotic Endothelial Dysfunction in COVID-19.” JCI Insight 6 (20). https://doi.org/10.1172/jci.insight.151527. https://insight.jci.org/articles/view/151527.