As the coronavirus disease 2019 (COVID-19) pandemic marches on, new antiviral agents effective against SARS-CoV-2 are urgently needed, despite a growing availability of vaccines. Remdesivir is still the only drug fully licensed for the treatment of affected individuals, but its effectiveness is controversial.
Iminosugar glucosidase inhibitors have their role in various viral diseases (e.g., hepatitis B and Ebola), as they prevent the folding process of a range of viral N-linked glycoprotein. Furthermore, they are competitive inhibitors of the host endoplasmic reticular (ER) glucosidases.
Since SARS-CoV-2 (but also the original SARS-CoV) utilize the angiotensin-converting enzyme 2 (ACE2) receptor, and both harbor highly N-glycosylated and essential spike polypeptides, a valid hypothesis is that SARS-CoV-2 would also be sensitive to ER glucosidase inhibition. In addition, they may potentiate anti-SARS-CoV-2 activity of direct antivirals, such as remdesivir.
Consequently, a research group from the University of Pennsylvania Perelman School of Medicine, Baruch S. Blumberg Institute and University of Texas Medical Branch at Galveston decided to explore validity to these assumptions by two iminosugar glucosidase inhibitors – NBDNJ (“Miglustat/Zavesca”) and ureido-N-hexyl deoxynojirimycin (BSBI-19029).
Methodological approach
In order to assess the ability of the aforementioned iminosugars NBDNJ and BSBI 19029 to successfully halt SARS-CoV-2 replication in tissue culture, researchers utilized NBDNJ obtained commercially, while BSBI-19029 was synthesized from tert-butyl-dimethyl-silyl protected bromohexyl-alcohol.
For antiviral analysis, human alveolar basal epithelial carcinoma cells (i.e., A549ACE2 cells) and both wild type SARS-CoV-2 and a stable mNeonGreen SARS-CoV-2 (icSARS-CoV-2-mNG) were used. The SARS-CoV-2 activity has been observed by direct imaging method with a Perkin Elmer EnVision multimode plate reader.
Moreover, since remdesivir is already in pervasive use for managing COVID-19, the researchers also wanted to determine whether combinations of NBDNJ or BSBI 19029 with remdesivir are also effective in tackling this infection.
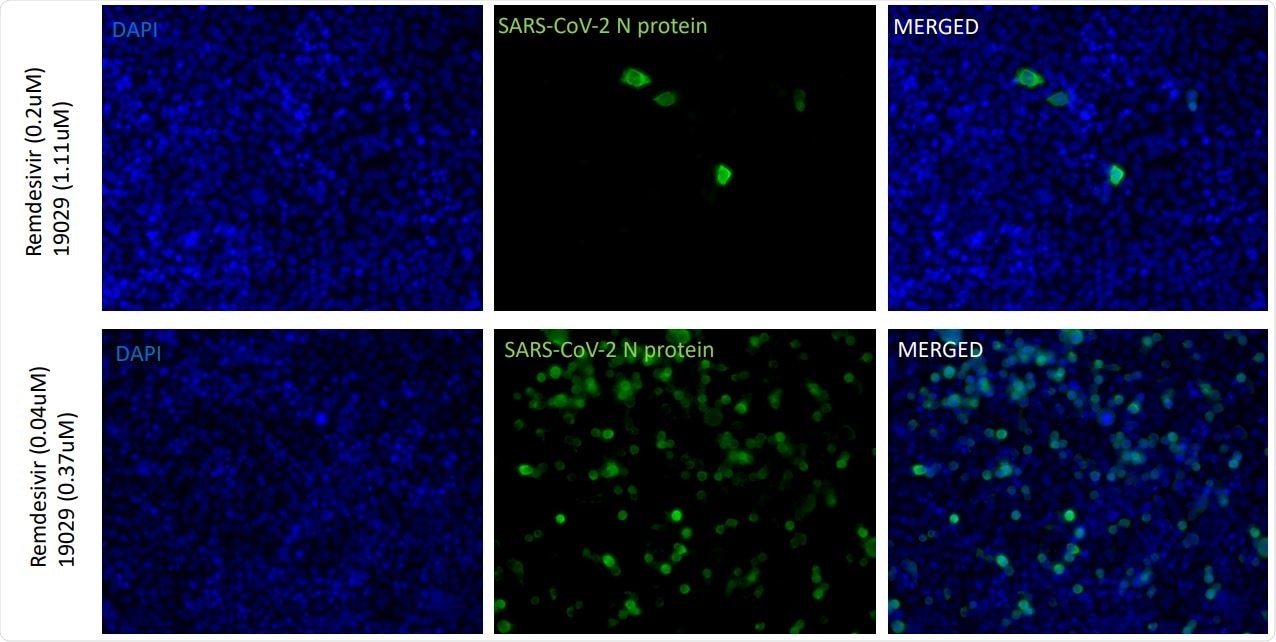
Combinations of NBDNJ and Remdesivir to suppress SARS-CoV-2. Human lung (carcinoma) A549ACE2 cells were infected with SARS-CoV-2 (USA-WA1/2020 strain) in the absence and presence of vary concentrations of drugs, and after 48 hours, stained with anti nucleocapsid antibody and images (20X) read in the blue filter to detect DAPI stain

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Eliminating the SARS-CoV-2 signal
In this study, 5 micromoles of remdesivir almost entirely suppressed the detectable SARS-CoV-2 signal. However, an encouraging finding was that NBDNJ and BSBI 19029 also displayed substantial antiviral activity, repressing most of the SARS-CoV-2 signal at 1000 micromoles and 5 micromoles, respectively.
In addition, remdesivir repressed icSARS-CoV-2-mNG in a dose-responsive manner, with a half-maximal inhibitory concentration (IC50) estimated to be approximately 2 nanomoles in this assay. Both NBDNJ and BSBI 19029 also repressed icSARS-CoV-2-mNG in a dose-responsive, non-cytotoxic manner.
The important finding was that the combination of NBDNJ or BSBI 19029 in certain micromolar doses with remdesivir almost completely eliminated the SARS-CoV-2 signal, without any evidence of antagonism between these drugs. Actually, it was quite the opposite – both compounds seem to potentiate the anti-SARS-CoV-2 activity of remdesivir.
New players in town?
Even though the exact concentrations of drugs used in this study may not directly correspond to concentrations that are needed in vivo, the results presented are rather promising. Also, taking into account the iminosugar’s mechanism of action, it was not surprising that iminosugars potentiated the antiviral activity of remdesivir, albeit the exact dosage still needs to be elucidated.
“Since NBDNJ is already a drug approved for human use, it is possible that evaluation of its value in managing SARS-CoV-2 could be explored in people fairly rapidly”, say study authors in this bioRxiv paper. “That it does not antagonize and may even potentiate remdesivir, which has been shown to be effective, but of limited therapeutic value, is particularly exciting”, they add.
In any case, more research endeavors on the topic are warranted, especially considering the potential of the aforementioned compounds to cause gastrointestinal distress when applied in higher doses and for protracted periods of time. However, the results presented here offer a ray of promise in broadening our therapeutic armamentarium against COVID-19.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.