Identifying markers that indicate severe COVID-19 disease trajectories could lead to early medical interventions and help reduce morbidity and mortality.
While Fab domains determine the binding specificity and the antibodies’ ability to block viral attachment to host cells, the Fc-Fc gamma receptor (FcγR) interactions induce a wide range of effector cell functions.
A better understanding of the structure of Fab and Fc domains is needed to predict COVID-19 disease trajectories
The structure of antiviral Fab and Fc domains decides if opsonized virus particles will be neutralized and how host cells engage with these particles. Although coronavirus disease 2019 (COVID-19 causes a wide range of clinical outcomes, it is still unclear why most people have none to mild symptoms while others have severe disease leading to hospitalization or death. Moreover, the impact of the kinetics and quality of the early immune response on disease trajectory is not well understood.
A better understanding of the Fab and Fc domain structures that predict various COVID-19 disease trajectories would enable early clinical interventions that will help reduce morbidity and mortality associated with COVID-19.
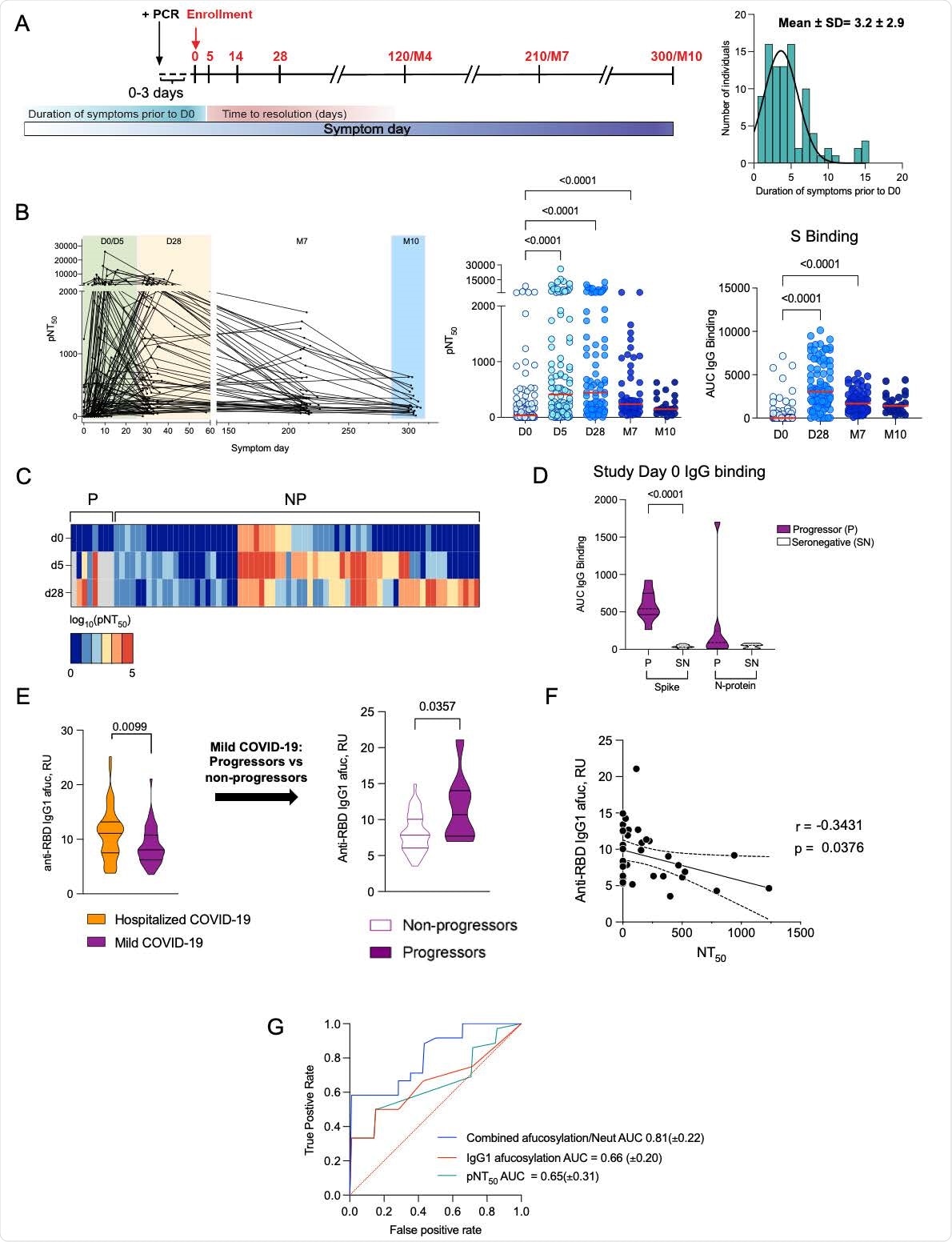
Low early neutralizing titers and elevated Fc afucosylation predict disease progression. (A) Longitudinal COVID-19 study timepoints, cohort characteristics and distribution of duration of symptoms in participants prior to enrolment. (B) The kinetics of neutralizing antibody response over time and half-maximal SARS-CoV-2 pseudovirus neutralizing titers (pNT50) and SARS-CoV-2 full length spike (S) binding IgG (AUC) at each time point ((D0, enrollment, n=109), day 5 (D5 n=108), day 28 (D28, n=92), month 7 (M7, n=65) and month 10 (M10, n=23)). The median values have been depicted with a red line. (C) Heatmap of longitudinal pNT50 data clustered according to the serum neutralization titer kinetics in progressors (P, n=8) and non-progressors (NP, n=68). (D) SARS-CoV-2 S and N-protein binding IgG (AUC) of progressors and seronegative (SN) historic sera samples. (E) Anti-RBD IgG1 Fc afucosylation (afuc) in patients who were hospitalized (n=52) and in those who were seropositive upon study enrollment in the longitudinal outpatient cohort (n=49). Pre-progression (at study enrollment) IgG1 Fc afucosylation levels in progressors (n=8) and non-progressors (n=41). (F) Correlation between SARS-CoV-2 IgG1 afucosylation and D0 neutralization titers. (Pearson’s correlation coefficient r=-0.3431, p=0.0376). (G) Mean ROC response and the area under the curve (AUC) with its standard deviation obtained using random forest classifier with 6-fold cross validation. P values (B) were calculated using Kruskal Wallis test with Dunn’s correction and in (D, E) using unpaired students t test.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Two separate disease trajectories
One trajectory involved early production of neutralizing antibodies resulting in mild disease, while the other trajectory was characterized by an initial phase of mild symptoms followed by rapid progression to severe disease. The latter trajectory was associated with the absence of early neutralizing antibodies with simultaneous production of afucosylated IgGs. Increased frequencies of monocytes that express the receptor for afucosylated IgGs, FcγRIIIa (CD16a), were also a predictor in patients with severe COVID-19 outcomes.
“Effective treatments that can prevent long-term symptoms of COVID-19 or that may halt the progression to severe COVID-19 are urgently needed to prevent mortality in communities that have not yet benefited from vaccination.”
In mechanistic studies, afucosylated immune complexes present in the lung induced an inflammatory infiltrate and cytokine production which was dependent on CD16a. In healthy subjects, mRNA vaccination induced the production of neutralizing antibodies enriched for Fc fucosylation and sialylation. These antibodies were distinct from those in both trajectories induced by infection.
Importance of combined Fab and Fc domain functions
Overall, these findings show the importance of combined Fab and Fc domain functions in eliciting an antiviral response and an early immune signature in patients with severe COVID-19 disease. They also have implications for new treatment strategies that target the FcγRIIIa pathways.
“Additional studies will be required to dissect which cells within the lung are the source of the factors that we observed in response to polyclonal afucosylated immune complexes.”
According to the authors, an important topic that need to be explored in future studies is the regulation of Fc fucosylation and the existence of genetic and/or modifiable determinants for this post-translational modification (PTM).
The differences in PTMs on IgGs induced by SARS-CoV-2 and mRNA vaccines show differential regulation on the basis of the antigen driving the response. However, it is clear that there are more determinants that drive the increased production of afucosylated IgGs in some people.
Overall, the results from the study confirm the crucial role of early neutralizing antibodies in offering protection against SARS-CoV-2. They also show that it is possible to identify severe disease trajectories early, which opens up promising avenues for early therapeutic intervention.
“An additional important topic for future studies is how Fc fucosylation is regulated and whether there are genetic and/or modifiable determinants of this post-translational modification.”

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Divergent early antibody responses define COVID-19 disease trajectories, Saborni Chakraborty, Joseph C. Gonzalez, Benjamin L. Sievers, Vamsee Mallajosyula, Megha Dubey, Bowie Yik-Ling Cheng, Kim Quyen Thi Tran, Srijoni Chakraborty, Arianna Cassidy, Steven T. Chen, Aanika Sinnott, Terri Gelbart, Yarden Golan, Mary Prahl, Upinder Singh, Seunghee Kim-Schulze, Robert Sherwood, Sheng Zhang, Thomas U. Marron, Sacha Gnjatic, Stephanie L. Gaw, Kari C. Nadeau, Miriam Merad, Prasanna Jagannathan, Gene S. Tan, Taia T. Wang, bioRxiv, 2021.05.25.445649; doi: https://doi.org/10.1101/2021.05.25.445649, https://www.biorxiv.org/content/10.1101/2021.05.25.445649v1