More than 170 million people have already been affected by the coronavirus pandemic known as Coronavirus disease 2019 (COVID-19). The causative agent of COVID-19 is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a highly infectious positive-stranded RNA virus.
Scientists have found that this virus severely affects primarily older patients with comorbidities, including diabetes, chronic lung diseases, obesity, and chronic kidney disease. It is more likely that these individuals will become severely infected by the virus. Therefore, there is an urgent need for effective therapeutics for this vulnerable group.
Recent studies have revealed that monoclonal antibody therapy can be effectively used to treat severely infected COVID-19 patients and can help lower the number of hospitalizations. These antibodies can neutralize SARS-CoV-2. SARS-CoV-2 variants have, however, raised concerns since some antibodies that may have been acquired via a prior COVID-19 infection or vaccination could be useless against these variants.
Monoclonal antibodies targeting evolutionarily conserved epitopes outside of the rapidly changing receptor-binding motif may be one strategy for treating COVID-19 disease. Due to the nonoverlapping resistance profile of these monoclonal antibodies, they are more effective against SARS-CoV-2 variants.
Recently, scientists have revealed that monoclonal antibody therapy sotrovimab effectively neutralizes SARS-CoV-2. Further, this engineered human monoclonal antibody can also neutralize many types of sarbecoviruses which include SARS-CoV, the causal agent of the SARS outbreak a couple of decades ago. Sotrovimab was previously known as VIR-7831.
A new study published on the medRxiv* preprint server hypothesized that a monoclonal antibody that has the capability to neutralize all sarbecoviruses could also be used to target a highly conserved epitope. This conserved sequence has a high probability of remaining unchanged in SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The current study has revealed that sotrovimab restores its function against the highly transmissible SARS-CoV-2 variants which were identified in South Africa (B.1.351), United Kingdom (B.1.1.7), California (B.1.427/B.1.429), and Brazil (P.1). Previous studies have reported the development of monoclonal antibodies for COVID-19 disease that could attach to the receptor-binding motif associated with the angiotensin-converting enzyme 2 (ACE2) receptor. However, this domain has a high possibility to get mutated and thereby, the monoclonal antibodies may remain ineffective against certain variants.
Researchers have explained that sotrovimab contains a two amino acid Fc modification, which is termed as LS and helps enhance half-life as well as the bioavailability in the respiratory mucosa. This modification enhances therapeutic concentrations for a longer period. In vitro study revealed that sotrovimab has potent effector functions that could be used in immune-mediated viral clearance.
The current study has conducted a preplanned interim analysis of the COVID-19 Monoclonal Antibody Efficacy Trial-Intent to Care Early (COMET-ICE) to determine the safety and efficacy of sotrovimab for the treatment of the vulnerable population with mild to moderate COVID-19 infection. Presently, the trial is closed for enrollment and the process of data collection, such as safety, efficacy, initial immunogenicity and laboratory data, is being undertaken.
Scientists have revealed in the COMET-ICE study, that one dose of sotrovimab, containing 500mg, has strongly decreased the rate of hospitalization and mortality in the group of individuals vulnerable to COVID-19 disease, in comparison with results attained using placebo.
The current study further reported that sotrovimab prevented one hospitalization for every seventeen high-risk patients with COVID-19 symptoms.
Researchers have observed that even hospitalized patients who received sotrovimab did not require admission to the intensive care unit. This was not so in patients who received the placebo. This result strongly indicated that sotrovimab treatment to vulnerable individuals with COVID-19 prevented the severity of the disease.
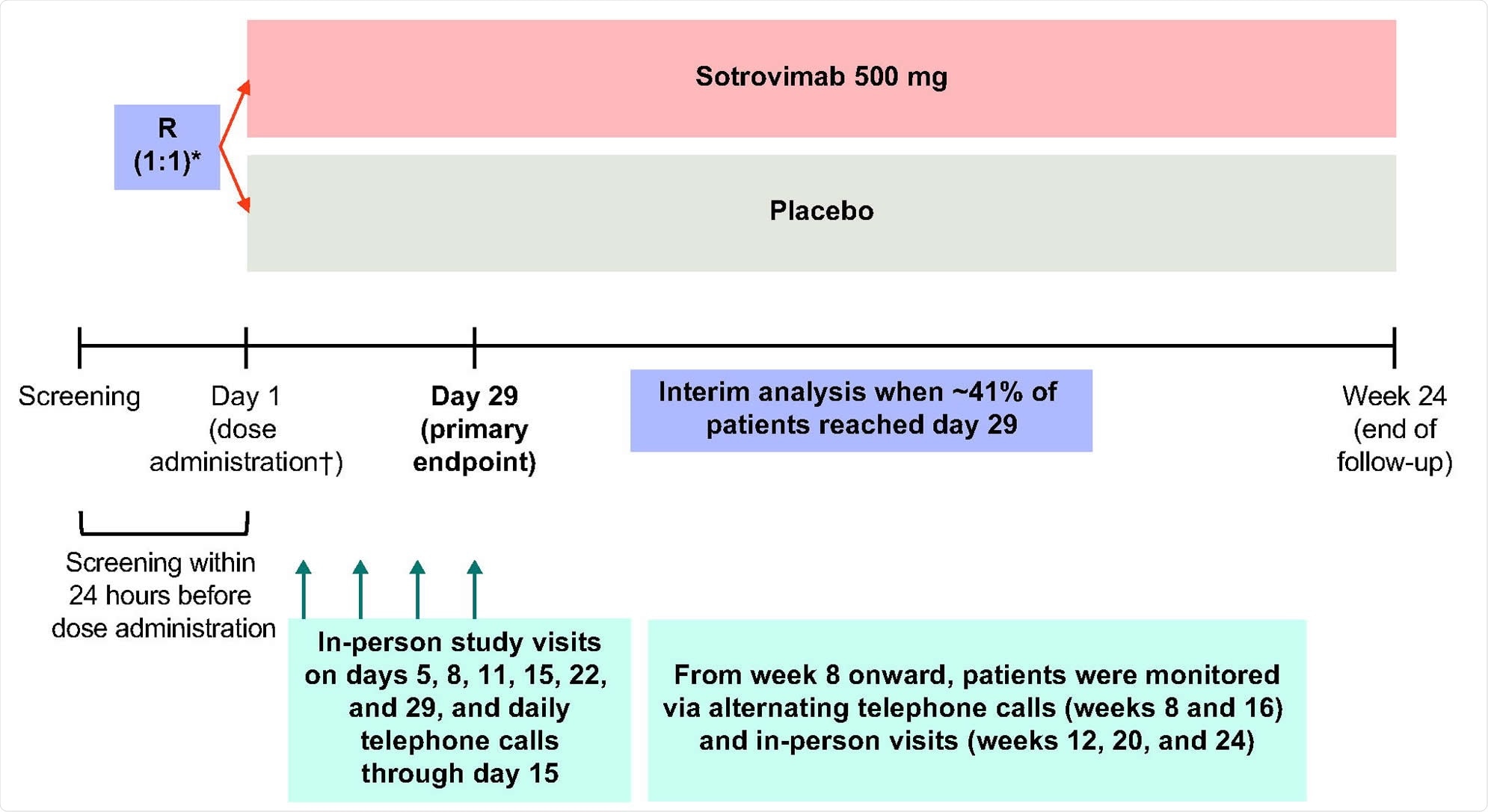
Study design. R denotes randomization. *Patients were stratified by age (≤70 vs. >70 years), symptom duration (≤3 days vs. 4-5 days), and region. †Study pharmacists reconstituted and dispensed all study medications within equal time frames to maintain blinding.
Another important aspect of this research is that it contained candidates who were vastly underrepresented ethnic groups in previous clinical trials. Researchers have reported that more than 60% of the study population consisted of COVID-19 patients who self-identified as Hispanic or Latino.
The present research revealed that sotrovimab was well-tolerated among the study population with no safety issues. Hence, researchers believe that sotrovimab can act as an important therapeutic against COVID-19, especially in conditions where vaccines are ineffective against SARS-CoV-2 variants or in the case of immunocompromised individuals who may not respond to a vaccine.
The current study evaluated more than 584,000 sequences in the Global Influenza Surveillance and Response System database (Global Initiative on Sharing All Influenza Data) and found that amino acid positions associated with sotrovimab epitope were 99.96% conserved in viruses. Further, sotrovimab can bind with currently authorized receptor-binding motif–targeted antibodies owing to its nonoverlapping resistance profile.
Despite the promising initial results, the study has a few limitations. Only three hospital admissions were found in the sotrovimab group, which is not enough to come to a conclusion. Further, the sample size that represents the safety dataset is moderate in size. Secondary and exploratory endpoint analyses have been omitted from the current interim report as the study is in progress, and thus, more data is needed to further highlight and establish the advantages of sotrovimab.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gupta, A. et al. (2021). Early Covid-19 Treatment With SARS-CoV-2 Neutralizing Antibody Sotrovimab.medRxiv 2021.05.27.21257096; doi: https://doi.org/10.1101/2021.05.27.21257096, https://www.medrxiv.org/content/10.1101/2021.05.27.21257096v1
- Peer reviewed and published scientific report.
Gupta, Anil, Yaneicy Gonzalez-Rojas, Erick Juarez, Manuel Crespo Casal, Jaynier Moya, Diego R. Falci, Elias Sarkis, et al. 2021. “Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab.” New England Journal of Medicine, October. https://doi.org/10.1056/nejmoa2107934. https://www.nejm.org/doi/10.1056/NEJMoa2107934.