Scientists from the Johns Hopkins University School of Medicine, USA, have recently developed an Antibody Display technology by grafting the second extracellular domain of tetraspanin12 (Tspan12EC2) and the receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein on the heavy chain complementarity-determining region 3 (CDR3). The study is currently available on the bioRxiv* preprint server.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
For the overall management of the ongoing coronavirus disease 2019 (COVID-19) outbreak, antibodies have played an immense role in a variety of aspects, including basic research, diagnostics, and therapeutics. The basic structure of an immunoglobulin G (IgG) molecule - a type of antibody - is composed of two heavy chains and two light chains. There are three CDRs in each antibody chain that form the antigen-binding site.
Recently, antibodies isolated from malaria-infected individuals have shown a novel structure wherein the extracellular Ig-like domain of leukocyte-associated immunoglobulin-like receptor 1 (LAIR1) is presented on the CDR3 of the antibody heavy chain.
Based on the LAIR1-containing antibody structure, the scientists have developed an Antibody Display technology by grafting the protein of interest onto the heavy chain CDR3 to produce bioactive chimera antibodies.
In the current study, the scientists have specifically selected two proteins, Tspan12EC2 and spike RBD, for grafting at the tip of the heavy chain CDR3.
Tetraspanin receptors play important roles in a variety of physiological functions, including cell-cell fusion, vascularization, and immune response. Of 33 members of the tetraspanin receptor family, tetraspanin12 plays a vital role in developing blood vessels in the brain and forming the blood-brain barrier. Moreover, tetraspanin12 is a co-activator of the Norrin-mediated beta-catenin signaling pathway. The second extracellular domain of tetraspanin12 is particularly important for the interactions with partner proteins or pathogens on the cell surface.
The spike RBD of SARS-CoV-2 plays the primary role in mediating viral entry by binding to the host cell angiotensin-converting enzyme 2 (ACE2) receptor. Because of the significant involvement in the viral entry process, the spike protein and spike RBD are considered the primary targets for vaccine or therapeutic antibody development.
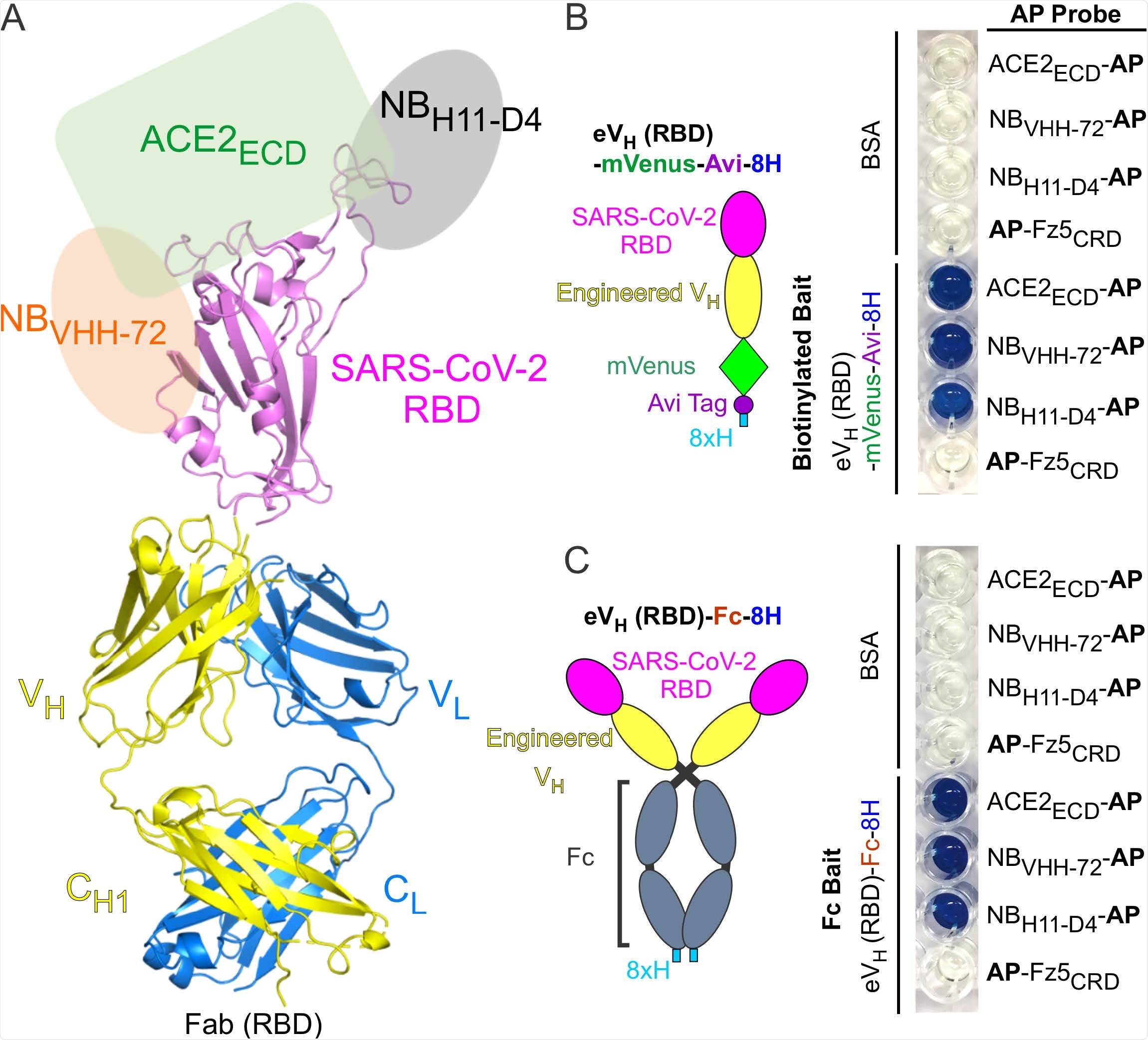
Antibody Display SARS-CoV-2 RBD (RBD-AD). (A) Molecular model of RBD-AD showing in the Fab format. The RBD (magenta) of SARS-CoV-2 S protein is displayed on the VH (yellow). A schematic diagram shows the binding sites on the RBD for ACE2ECD (green), NBVHH-72 (orange), and NBH11-D4 (grey). (B) A diagram of the RBD of SARS-CoV-2 S protein is displayed on the engineered VH fused to a mVenus, an Avi tag, and an 8xH tag at the C-terminus. For the AP based binding assay, biotinylated baits were immobilized on streptavidincoated wells. Bound AP fusion proteins (probes) were visualized with BluePhos phosphatase substrate solution. BSA and Fz5CRD fusion proteins are used as negative controls. (C) Diagram of SARS-CoV-2 RBD-containing engineered VH fused an 8xH tagged human Fc. FC baits were captured on protein-G coated wells and incubated with AP fusion proteins (probes). The bait and probe interactions were detected as in panel (B).
Important observations
To determine whether the Antibody Display Tspan12EC2 is correctly folded and retaining the biological activity, the scientists assessed the binding of recombinant Tspan12EC2 to Norrin using protein-protein interaction assays. The findings revealed that the Antibody Display Tspan12EC2 interacted with Norrin with similar efficiency as Tspan12EC2. This indicates that Tspan12EC2 can be grafted onto the heavy chain CDR3 without disrupting Norrin binding ability.
Furthermore, for functional characterization of Antibody Display RBD, they generated recombinant ACE2 and two anti-RBD nanobodies. In addition, they prepared bait proteins for the Antibody Display RBD, which included Fab-RBD and engineered VH domain-RBD constructs containing a C-terminal fluorescent protein and biotinylated peptide tag; and an engineered VH domain-RBD construct containing the human IgG Fc fusion at the C-terminus.
By conducting protein-protein interaction assays using these constructs, they observed that the Antibody Display RBD interacted strongly and specifically with ACE2 and neutralizing nanobodies. This indicates that the SARS-CoV-2 spike RBD grafted onto the heavy chain CDR3 is capable of maintaining its biological activity.
Study significance
The study describes the design and production of an Antibody Display platform to graft biologically active proteins of interest. By grafting the proteins of interest onto the beta-strands G and F of the heavy chain, the Antibody Display technology provides a number of potential benefits, including generation of conformationally controlled chimeric protein and up-gradation of current antibody engineering machineries for producing bispecific and chimeric antibodies.
Given the critical involvement of tetraspanin12 in the brain vasculature development and tumorigenesis, the Antibody Display Tspan12EC2 can serve as a valuable platform for studying the pathophysiology of various brain disorders.
Similarly, the Antibody Display RBD may be used as a potential platform to induce antibody-mediated immune responses against SARS-CoV-2.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.