The causative agent of the coronavirus disease 2019 (COVID-19) – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – was first detected in Wuhan, China, in December 2019. To date, the virus has spread to over 192 countries and territories, infected over 173.6 million people and caused over 3.7 million deaths.
The unprecedented speed with which vaccines against SARS-CoV-2 had been developed was due to the culmination of years of prior research.
Recombinant viral vectors, protein-based vaccines, nucleic acid-based vaccines, and inactivated/attenuated viruses have been the platforms used to develop the current COVID-19 vaccines. These vaccines have been thoroughly scrutinized for safety and efficacy before being authorized for use, and this has encompassed environmental risk assessment.
In a recent review, published in the journal Vaccines, researchers in Belgium look at the recombinant viral vector-vaccine candidates that are primarily against SARS-CoV-2 still in development and discuss their features from an environmental risk point of view.
The researchers presented the general principles of the environmental risk assessment (ERA) and elaborated on the key features of several recombinant viral vector COVID-19 vaccine candidates.
In the review, the researchers presented a detailed table listing the viral vector-based vaccines against SARS-CoV-2 that are in clinical development or already authorized for use in the European Union, including references. It provided information on the developer or manufacturer of the COVID-19 vaccine, the genetic modifications of the vector, the inserted gene sequences there, the route of administration of the vaccine and the clinical stage of the vaccine trial.
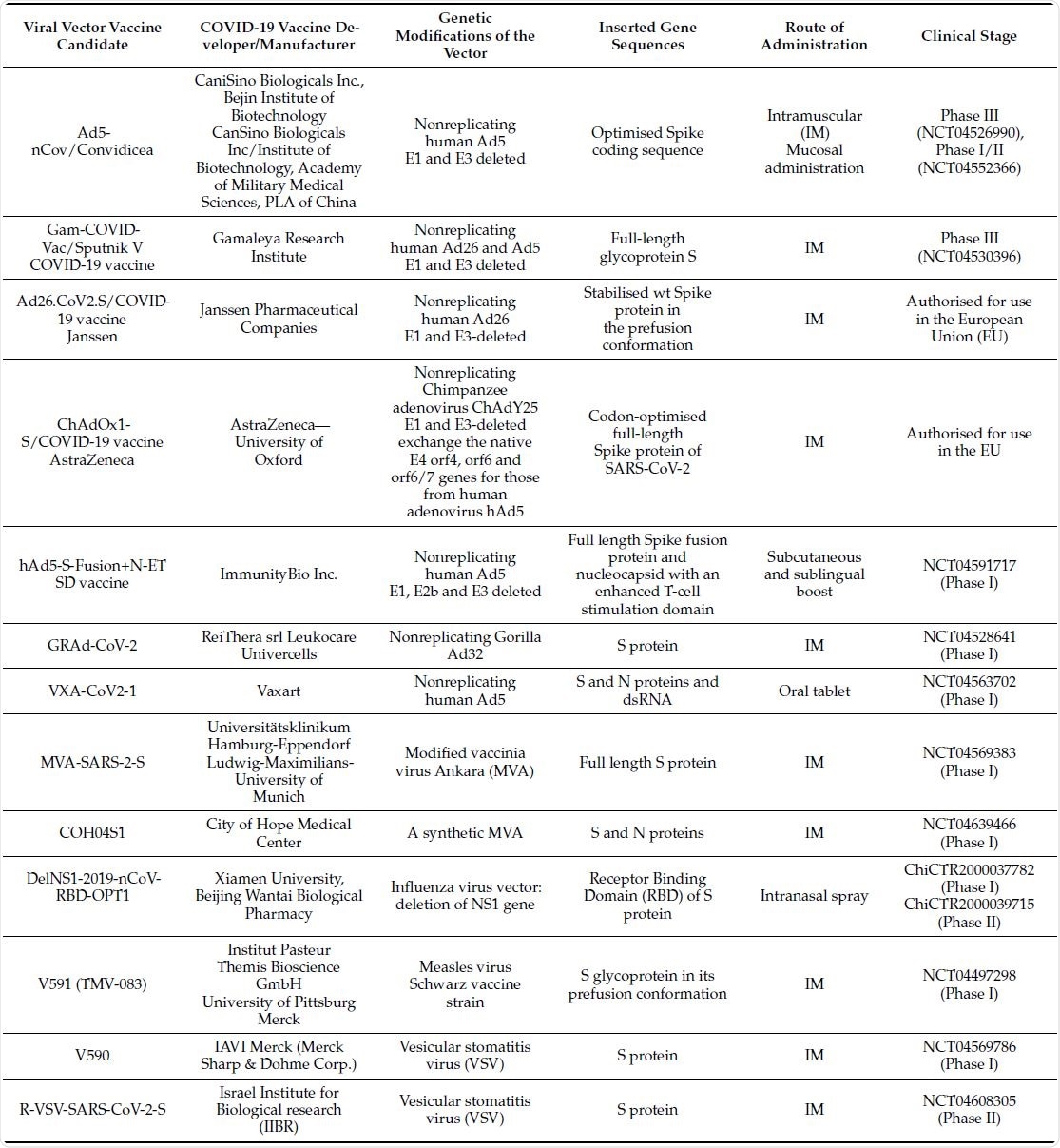
Viral vector based vaccines against SARS-CoV-2 that are in clinical development or already authorised for use in the European Union. Image Credit: Original Article / Vaccines
All vaccines undergo clinical trials and regulations to ensure that the vaccines comply with relevant requirements regarding their efficacy and safety (for the human subjects) and their quality control.
Because some of the vaccines based on the recombinant viral vectors are also considered as genetically modified organisms (GMOs), some of the additional regulatory requirements have their legal basis in Directive 2001/18/EC and aim to assess aspects related to potential risks for human health and the environment, including animals, plants and microorganisms. This is called the “environmental risk assessment (ERA).”
An ERA assesses potential hazards associated with the candidate on human health, particularly with a focus on individuals other than patients or those who are vaccinated, and the environment at large. The risk is estimated by combining the probability of occurrences and the magnitude of their consequences.
While the ERA must be conducted on a case-by-case basis, the researchers highlighted that for a recombinant viral vector, the ERA should take into account the characteristics of the viral vector backbone and the properties of the inserted gene sequence(s) and the gene product(s).
They discussed the elements related to the viral vector-based vaccines against SARS-CoV-2 to assess these in the context of an environmental risk assessment (ERA), which involved an examination of the viral vector backbone and the characteristics of the inserted gene sequences. They tabulated a concise summary of how ERA could be applied to viral vector-based vaccines against SARS-CoV-2.
The researchers discussed the role of the sequences in the recombinant viral vector COVID-19 vaccines and how they are strategized to stay stable and effectively activate the immune system. They cautioned the potential impact of altered nucleic acid sequences or altered amino acids in the S protein on the biodistribution or host range profile of recombinant viral vectors, particularly when the protein is expressed on the surface of the virion.
Because the S protein is the main determinant for cross-species infection events and may play a role in host tropism, they highlighted its role for ERA considerations when it is also expressed on the surface of the recombinant viral vector.
It is also possible that the viral vectors may interact with other viruses (maybe other endemic coronaviruses in this case) that present a high degree of homology. Depending on several factors, this may lead to recombination events and result in the formation of novel uncharacterized chimeric viruses.
The researchers write:
For virions expressing surface-exposed heterologous proteins playing a role in cell attachment, the collection of specific data on tropism, biodistribution and shedding properties becomes key to a good understanding of their in vivo behaviour.”
The researchers elaborated on the environmental risk assessment of recombinant viral vector vaccines against SARS-CoV-2. They discussed the replication-deficient viral vectors, such as the Adenoviral Vectors, Modified Vaccinia Virus Ankara (MVA) Vectors, Recombinant Influenza Virus Vectors; and the Replication Competent Viral Vectors, such as the Live-Attenuated Measles Virus Vector, Vesicular Stomatitis Virus (VSV)-Vectors. They reviewed the synthesis of each vector, immune responses, host range, cellular tropism and biodistribution, the potential for genome interaction and recombination, shedding extent (if any) and the route of administration of the vaccine, among other factors.
Building on the experience gained with some viral platforms and/or the collection of data for other emerging viral vectors, the case-by-case principle as embedded in the ERA methodology and illustrated in this article should provide a solid basis to guarantee a scientifically sound, adequate and proportionate approach, the researchers conclude.