Why is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19 (coronavirus disease 2019), biased towards infecting the aged population? A recently published study by Italian scientists linked aging, DNA damage, telomere shortening, and increased levels of angiotensin-converting enzyme 2 (ACE2) to this relationship.
To better understand the modulation of the ACE2 receptors and the molecular mechanisms involved, the researchers studied the expression of ACE2 in mouse and human lungs at different ages. Publishing their results on the preprint server (not peer-reviewed) bioRxiv*, they found that the ACE2 promoter is dependent on the DNA damage response; the ACE2 is regulated at the transcription level.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Compared to young mice (2-3 months), the ACE2 mRNA levels were increased in old mice (22-23 months). In human lung tissue samples, the researchers found the ACE2 protein expression levels higher in older subjects (60-80 years old) compared to the young ones (20-35 years old). Further, the researchers showed that the ACE2 was predominantly expressed in ATII pneumocytes, and it increased with aging.
Previous studies have shown that the severity of SARS-CoV-2 infection correlates with high rates of alveolar epithelial type II (ATII) cell infection. The results are consistent with similar analyses performed on non-human primates.
Because aging is associated with telomere shortening and damage in several tissues, to study the association between aging and ACE2 modulation, the researchers monitored the ACE2 mRNA levels in human fibroblasts (BJ) and human bronchial epithelial cells (HBECs) at different population doublings. Both these cell types do not have telomere maintenance mechanisms and are characterized by progressive telomere shortening upon proliferation. Compared to the early cell passages, both the cells expressed increased levels of mRNA.
A mouse model lacking the RNA component of telomerase (Terc-/-) is similar to features of human aging in different tissues, including lungs. The researchers observed a consistent increase of ACE2 expression in these mice compared to the wild types.
Further, they tested the role of the DNA damage response (DDR) pathway and observed a correlation between DDR increase and the ACE2 mRNA levels. Interestingly, they also checked if ionizing radiation-induced DDR activation drove a similar increase of ACE2 mRNA levels. The researchers confirmed, “this study indicated that while telomeric DDR activation is the most likely event increasing ACE2 levels during physiological aging, additional events activating DDR could also contribute.”
They also demonstrated this in vivo - the activation of DDR at the telomere leads to increased Ace2 expression.
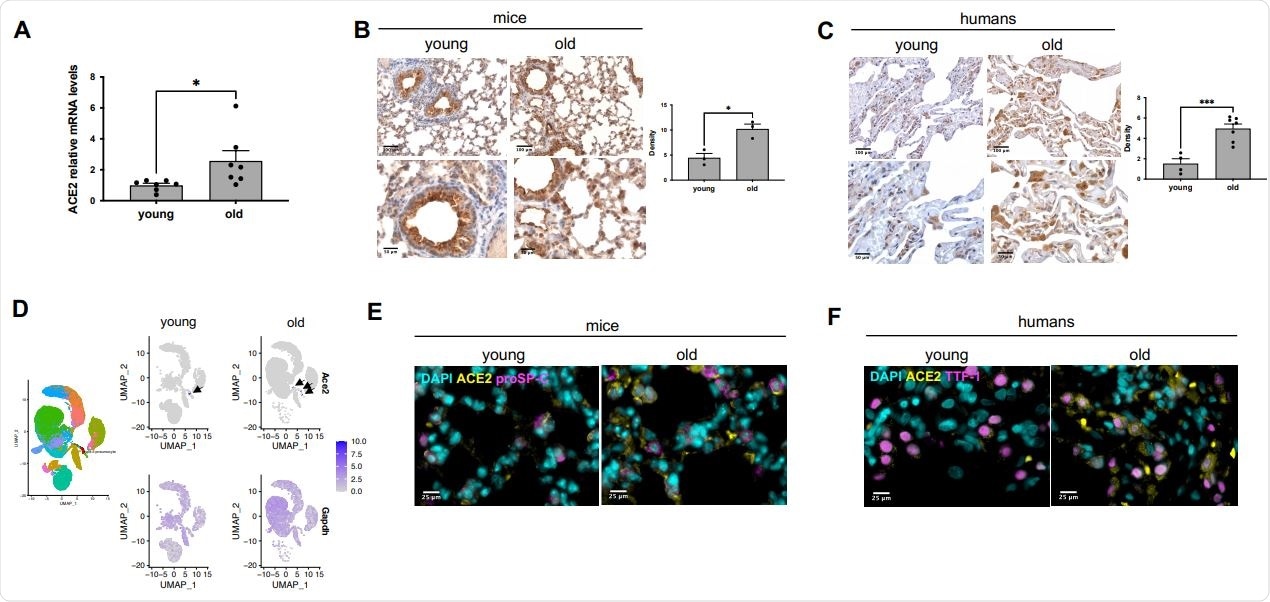
ACE2 expression increases during ageing in mouse and human lungs
To determine whether the ACE2 promoter could respond to DDR activation, they performed an in silico analysis. They identified the transcription factors DNA binding motifs significantly enriched in the promoter region of ACE2. “Gene set enrichment analysis of the top 100 transcription factors potentially associated with the ACE2 promoter, revealed an over-representation of pathways possibly related to the DNA damage response,” observed the researchers in the paper. They also confirmed this experimentally.
When the DDR foci formation is prevented, the ACE2 expression is reduced. Importantly, the researchers also showed that the telomeric antisense oligonucleotides (tASOs) are effective in controlling ACE2 levels in vivo in mouse tissues, including the lung.
“Taken together, these results clearly demonstrate that the expression of ACE2, the SARS-CoV-2 receptor is directly modulated by the activation of the DDR pathway at the transcriptional level and that telomere dysfunction is a physiological event able to engage the DDR pathways modulating ACE2 levels.”
While COVID-19 ranges from asymptomatic infection to multi-organ failure and death, the severity of the infection is correlated with the age of patients. Thus, along with comorbidities, age is an important risk factor that influences the outcome. This study is significant in establishing a correlation between aging, DNA damage, telomere shortening, transcriptional control of ACE2 receptors - the underlying mechanisms.
Interestingly, based on this study and the fact that ASOs are established medicines approved for several disorders and also that the telomeric DDR increases with age in normal and pathological conditions (Rossiello et al., Nature Cell Biology, under submission), the researchers suggest that upon additional validation, tASOs can have the potential as therapeutic agents to reduce susceptibility to SARS-CoV-2 infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
DNA damage response at telomeres boosts the transcription of SARS-CoV-2 receptor ACE2 during aging, Sara Sepe, Francesca Rossiello, Valeria Cancila, Fabio Iannelli, Valentina Matti, Giada Cicio, Matteo Cabrini, Eugenia Marinelli, Busola Alabi, Alessia di Lillo, Arianna Di Napoli, Jerry W Shay, Claudio Tripodo, Fabrizio d'Adda di Fagagna, bioRxiv 2021.06.09.447484; doi: https://doi.org/10.1101/2021.06.09.447484, https://www.biorxiv.org/content/10.1101/2021.06.09.447484v1
- Peer reviewed and published scientific report.
Sepe, Sara, Francesca Rossiello, Valeria Cancila, Fabio Iannelli, Valentina Matti, Giada Cicio, Matteo Cabrini, et al. 2021. “DNA Damage Response at Telomeres Boosts the Transcription of SARS‐CoV‐2 Receptor ACE2 during Aging.” EMBO Reports 23 (2). https://doi.org/10.15252/embr.202153658. https://www.embopress.org/doi/full/10.15252/embr.202153658.