COVID-19, one of the deadliest pandemics in history, has been associated with more than 176 million infections and some 3.82 million confirmed deaths as of 16 June 2021.
Most countries have put in place phased vaccine distribution plans that prioritize those at the highest risk of complications, such as the elderly and those most exposed and at high risk for transmission, such as medical personnel.
Several COVID‑19 vaccines have demonstrated efficacy as high as 95% in preventing symptomatic COVID‑19 infections. Globally 17 vaccines are authorized by at least one national regulatory authority for public use. In total, as of March 2021, 308 vaccine candidates are in various stages of development.
Annual influenza vaccination is also a part of the public health recommendations in several countries as a preventative strategy to curb the seasonal epidemic that affects millions of people each year.
Globally, more than 2.4 billion doses of COVID-19 vaccine have been administered, and this ongoing mass vaccination campaign will undoubtedly coincide with seasonal flu vaccination campaigns.
The timing of booster COVID-19 vaccine doses in many countries will likely overlap with the 2021–2022 flu season in many settings. In addition, many other countries could still be administering the first doses of COVID-19 vaccines during the flu season.
There are currently insufficient data regarding the co-administration of COVID-19 vaccines with other vaccines. In order to formulate effective public health policies, it is essential to understand how co-administration affects safety and immune responses.
This is even more important in older adults as immunosenescence may leave them more vulnerable to seasonal influenza infection, related complications, and mortality and reduce their immune responses to regular influenza vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Evaluating the safety and efficacy of the NVX-CoV2373 vaccine when co-administered with the flu vaccine
Researchers from the UK recently reported the results of a sub-study that evaluates the safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine when co-administered with an approved seasonal influenza vaccine.
This sub-study was part of a phase 3 randomized UK trial of the safety and efficacy of the NVX-CoV2373 vaccine. Approximately 400 participants of the main trial who met the sub-study entry criteria and had no contraindications to the flu vaccine were chosen for the sub-study. This work is published on the medRxiv* preprint server prior to the peer review process.
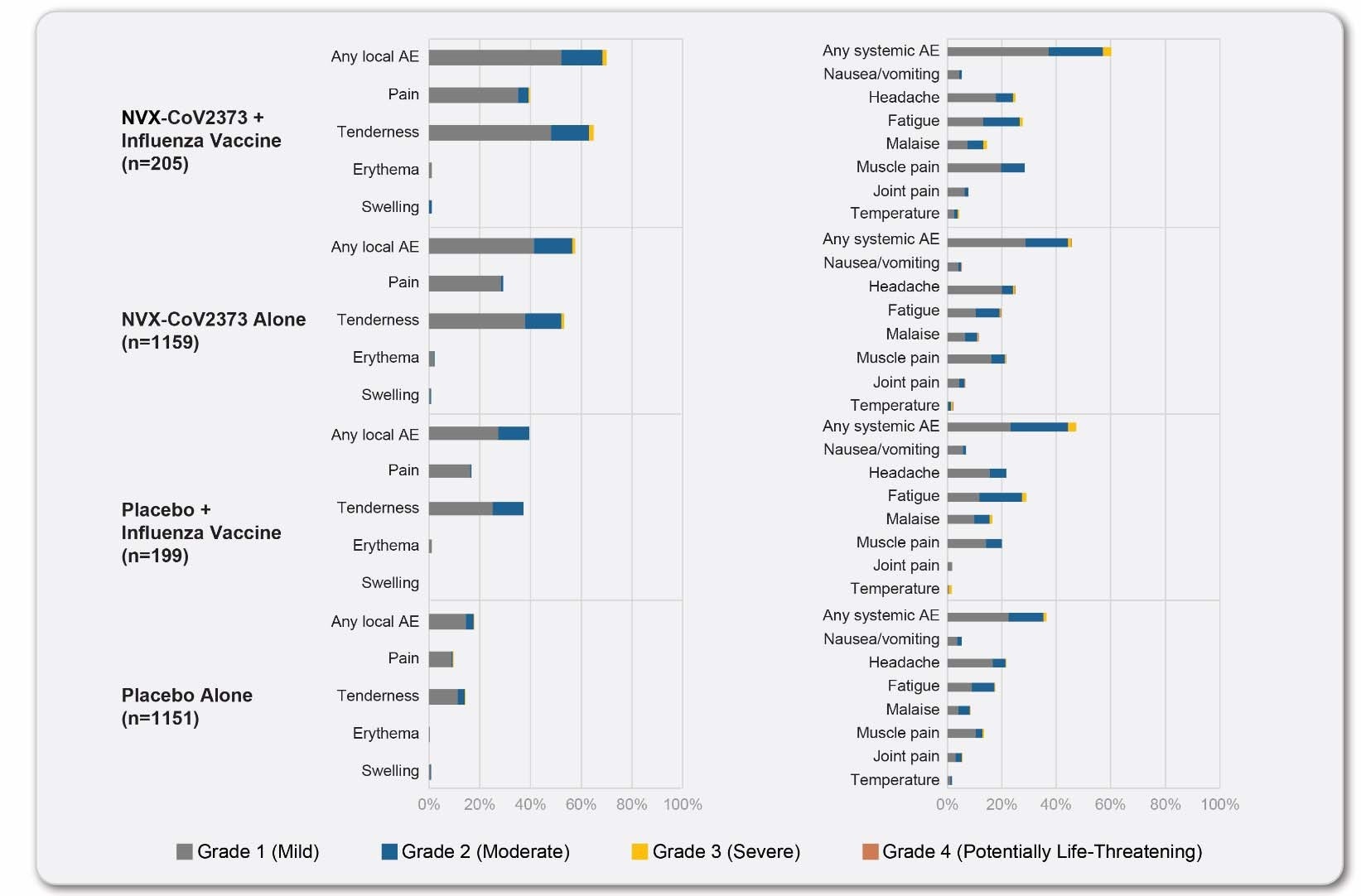
Reactogenicity data from participants in the influenza vaccine co-administration sub-study and participants in the reactogenicity cohort population after dose 1: local and systemic. The percentage of participants in each treatment group with solicited local and systemic adverse events during the 7 days after each vaccination is plotted according to the maximum toxicity grade (mild, moderate, severe, or potentially life-threatening) in participants included in the seasonal influenza vaccine sub-study and those included in the reactogenicity cohort.
The sub-study participants were randomized in a 1:1 ratio to receive NVX-CoV2373 (n=217) or placebo (n=214) and were given an approved, age-appropriate influenza vaccine along with the first dose of NVX-CoV2373.
Reactogenicity to the vaccines was determined with the help of an electronic diary for 7 days after vaccination. The participants were also monitored for medically attended AEs (MAAEs), unsolicited adverse events (AEs), and serious AEs (SAEs). In addition, SARS-CoV-2 anti-spike IgG and Influenza hemagglutination inhibition assays were performed. The authors also assessed vaccine efficacy against PCR-confirmed, symptomatic COVID-19 and made comparisons between sub-study and main study participants.
Findings show that concomitant vaccination did not alter the immune response to the seasonal influenza vaccine
The participants of the sub-study were younger and more racially diverse compared to the main study participants. They also had fewer comorbid conditions. Reactogenicity events that were more common in the co-administration group were tenderness or pain at the injection site, fatigue, and muscle pain.
Unsolicited AEs, MAAEs, and SAEs were low and balanced among the two groups. While co-administration of the vaccines did not result in any change to the immune response to the seasonal influenza vaccine, a reduction was noted in the antibody response to the NVX-CoV2373 vaccine. While vaccine efficacy in the main study was 89.8%, efficacy in the sub-study was 87.5%.
Study suggests that co-administration of COVID and flu vaccines may be a viable immunization strategy
According to the authors, this is the first study demonstrating the safety, efficacy, and immunogenicity of a COVID-19 vaccine when co-administered with seasonal flu vaccines. The study's findings suggest that co-administration of COVID and flu vaccines is fairly safe and might be a viable immunization strategy.
Although no specific comparative immunogenicity endpoints were specified in this exploratory sub-study, the authors found no evidence for the COVID-19 vaccine interfering with the QIVc influenza vaccine. This study offers crucial information that can help guide the national immunization policy decision-making on concomitant use of COVID-19 vaccines with influenza vaccines. The authors hope that future clinical trials and studies of COVID-19 vaccines include safety and immunogenicity data on co-administration with other common pediatric and adult vaccines.
"More research on the concomitant vaccination of COVID-19 and influenza vaccines is needed, especially in those >65 years of age, to help guide national immunization policy on this critical issue."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Safety, Immunogenicity, and Efficacy of a COVID-19 Vaccine (NVX-CoV2373) Co-administered With Seasonal Influenza Vaccines, Seth Toback, Eva Galiza, Catherine Cosgrove, James Galloway, Anna L. Goodman, Pauline A. Swift, Sankarasubramanian Rajaram, Alison Graves-Jones, Jonathan Edelman, Fiona Burns, Angela M. Minassian, Iksung Cho, Lakshmi Kumar, Joyce S. Plested, E. Joy Rivers, Andreana Robertson, Filip Dubovsky, Greg Glenn, Paul T. Heath, medRxiv, 2021.06.09.21258556; doi: https://doi.org/10.1101/2021.06.09.21258556, https://www.medrxiv.org/content/10.1101/2021.06.09.21258556v1
- Peer reviewed and published scientific report.
Toback, Seth, Eva Galiza, Catherine Cosgrove, James Galloway, Anna L Goodman, Pauline A Swift, Sankarasubramanian Rajaram, et al. 2021. “Safety, Immunogenicity, and Efficacy of a COVID-19 Vaccine (NVX-CoV2373) Co-Administered with Seasonal Influenza Vaccines: An Exploratory Substudy of a Randomised, Observer-Blinded, Placebo-Controlled, Phase 3 Trial.” The Lancet Respiratory Medicine, November. https://doi.org/10.1016/S2213-2600(21)00409-4. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00409-4.