Researchers in the United States have demonstrated the effectiveness of early treatment with REGEN-COV™ at preventing symptomatic disease among people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
The antibody combination treatment has already been shown to be effective at reducing the risk of hospitalization and death among individuals with symptomatic COVID-19.
Now, Meagan O’Brien from the University of Pennsylvania in Philadelphia and colleagues have shown that individuals infected with SARS-CoV-2 who have not yet developed any symptoms also benefit from receiving REGEN-COV, with the treatment reducing the risk of symptomatic disease by 31.5%.
“Subcutaneous REGEN-COV 1200mg prevented progression from asymptomatic to symptomatic infection, reduced the duration of high viral load and symptoms, and was well tolerated,” writes O’Brien and colleagues.
The team suggests that using REGEN-COV as a complement to vaccination may dramatically reduce the overall reservoir of virus in the community and decrease disease severity and healthcare utilization among infected individuals.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 incubation period complicates control of the virus
Since the COVID-19 outbreak first began in late December 2019, the causative agent SARS-CoV-2 has caused more than 176 million infections and more than 3.8 million deaths globally.
Despite widespread vaccination and the emergency use of monoclonal antibody combinations, approximately 70,000 cases of COVID-19, 6,000 hospitalizations, and 1,000 deaths occur in the United States every month.
The SARS-CoV-2 incubation period is highly variable (ranging from 2 to 14 days), which complicates the control of virus transmission, as high viral loads and viral shedding often characterize this period.
Furthermore, current evidence suggests that at least one-third of all infections are asymptomatic, with high levels of viral shedding potentially driving the ongoing transmission.
“Easy-to-administer antiviral treatments that reduce SARS-CoV-2 burden early in the pre-symptomatic phase or in asymptomatic individuals could reduce the incidence of moderate and severe COVID-19 and blunt ongoing transmission,” write the researchers.
REGEN-COV comprises two neutralizing monoclonal antibodies – casirivimab and imdevimab – that bind distinct epitopes on the SARS-CoV-2 spike protein receptor-binding domain (RBD) and block virus entry into host cells. The spike RBD initiates the initial stage of the infection process when it binds to the human host cell receptor angiotensin-converting enzyme 2 (ACE2).
Intravenous administration of this two-antibody combination has already been shown to significantly reduce the risk of hospitalization and death in outpatients with symptomatic COVID-19.
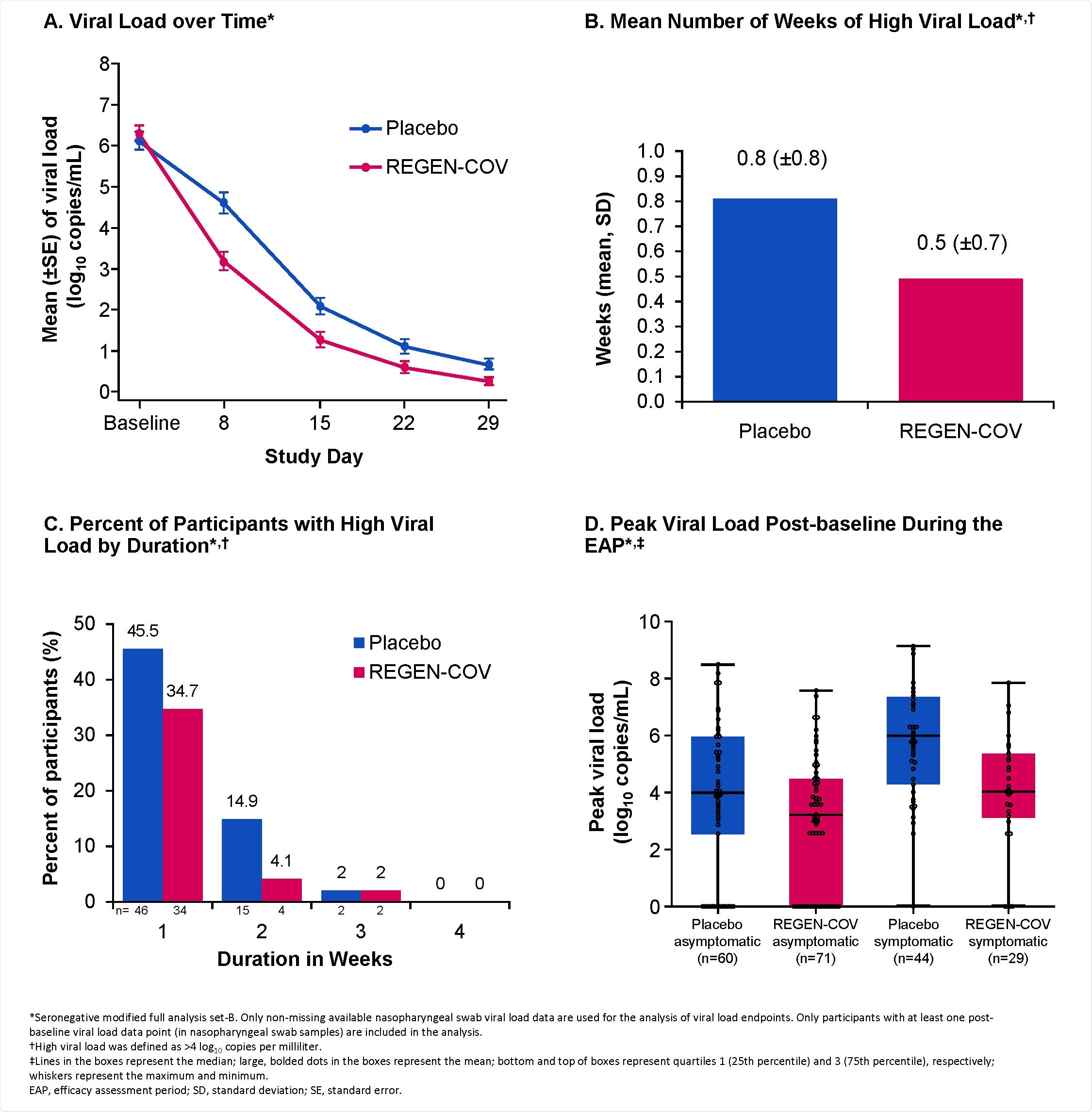
Reduction in Viral Load with REGEN-COV. A. Viral Load over Time* B. Mean Number of Weeks of High Viral Load*,† C. Percent of Participants with High Viral Load by Duration*,† D. Peak Viral Load Post-baseline During the EAP*,‡ *Seronegative modified full analysis set-B. Only non-missing available nasopharyngeal swab viral load data are used for the analysis of viral load endpoints. Only participants with at least one post-baseline viral load data point (in nasopharyngeal swab samples) are included in the analysis. †High viral load was defined as >4 log10 copies per milliliter. ‡Lines in the boxes represent the median; large, bolded dots in the boxes represent the mean; bottom and top of boxes represent quartiles 1 (25th percentile) and 3 (75th percentile), respectively; whiskers represent the maximum and minimum. EAP, efficacy assessment period; SD, standard deviation; SE, standard error.
What did the current study involve?
For the study, 314 individuals (aged 12 years or more) who were recently infected with SARS-CoV-2 through contact with a household index case, but had not yet developed any symptoms, were randomly assigned to receive a single dose of REGEN-COV 1200mg or placebo by subcutaneous injection.
The primary endpoint was the proportion of infected participants without evidence of previous immunity who subsequently developed symptomatic COVID-19 during a 28-day efficacy assessment.
The trial took place at 112 sites in the United States, Romania, and Moldova. It was managed jointly by Regeneron, the COVID-19 Prevention Network, and the National Institute of Allergy and Infectious Diseases.
What did the study find?
Subcutaneous treatment with REGEN-COV 1200mg reduced the risk of progression from asymptomatic to symptomatic disease by 31.5%, compared with placebo.
Viral load declined more rapidly in the REGEN-COV versus placebo groups, with a mean difference in viral load of -1.5 log10 copies/mL by day eight.
The number of weeks for which participants had a high viral load (more than 4 log10 copies/mL) was reduced by 39.7% among the REGEN-COV-treated participants (48 weeks), compared with among placebo recipients (82 weeks).
“From a public health perspective, treatment of early asymptomatic individuals may decrease the overall population burden of high viral load carriage, reducing the reservoir for potential further transmission and virus mutation,” says O’Brien and colleagues.
The number of weeks for which participants had symptoms was reduced by 45.3% in the REGEN-COV group (90 weeks), compared to the placebo group (170 weeks).
This corresponded to a 5.6-day reduction in the mean duration of symptoms for each symptomatic participant (21.7 days for REGEN-COV versus 27.3 days for placebo).
REGENCOV has an acceptable safety profile
Among those who received REGEN-COV, no COVID-19-related hospitalization or emergency room (ER) visits occurred. In contrast, three placebo recipients visited ER, one was hospitalized, and two visited ER and were subsequently hospitalized.
The proportion of participants who had one or more treatment-emergent adverse events was 48.1% among placebo recipients, compared with 33.5% for those receiving REGEN-COV.
“Subcutaneous administration of REGENCOV is efficacious with an acceptable safety profile, thus potentially providing substantial benefits by avoiding the healthcare resources necessary for an intravenous infusion,” writes the team.
There is rationale for using REGENCOV in various settings
O’Brien and colleagues say there is a rationale for using REGENCOV in various settings, from infection prevention to early treatment of asymptomatic individuals and symptomatic, high-risk COVID-19 outpatients.
“As a complement to vaccines, widespread utilization in these settings – which can be more easily accomplished with a convenient subcutaneous regimen – may dramatically reduce the overall reservoir of virus in the community, as well as decrease disease severity and healthcare utilization in infected individuals,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
O’Brien M, et al. Subcutaneous REGEN-COV Antibody Combination in Early SARS-CoV-2 Infection. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.14.21258569, https://www.medrxiv.org/content/10.1101/2021.06.14.21258569v1
- Peer reviewed and published scientific report.
O’Brien, Meagan P., Eduardo Forleo-Neto, Neena Sarkar, Flonza Isa, Peijie Hou, Kuo-Chen Chan, Bret J. Musser, et al. 2022. “Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection.” JAMA, January. https://doi.org/10.1001/jama.2021.24939. https://jamanetwork.com/journals/jama/fullarticle/2788256.