Researchers found antibody levels in mRNA vaccinated individuals varied with age, gender, and previous coronavirus disease (COVID-19) infection. With several vaccines now being approved for combating COVID-19, the attention is now turning to understand how they are working in real-life scenarios. One type of approved vaccine is based on mRNA technology, and two slightly different types are approved for use, one made by BioNtech/Pfizer and the other by Moderna.
Clinical trials of the BioNtech/Pfizer BNT162b2 vaccine have shown an efficacy of 95% in preventing symptomatic COVID-19 about a week after the second dose. Similar efficacy of about 94% was seen with the Moderna vaccine. Studies of the real-world vaccination in Israel have provided more evidence for the efficacy of the BNT162b2 vaccine.
Vaccine efficacy has a strong correlation with neutralizing antibodies, but it is not easy to measure neutralizing antibodies on a large scale. Binding assays to the spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have a good correlation with neutralizing antibodies and can be used to investigate the immune response to vaccines at a large scale. Assessing immune response to vaccines can help predict vaccine effectiveness in people who were not included in clinical trials, such as immunocompromised individuals.
It is yet not known how long protection lasts after vaccination. Antibodies to other coronaviruses, including after natural infection of SARS-CoV-2, decrease with time. After natural infection with SARS-CoV-2, protection lasts for at least seven months.
Results of Study. Image Credit: https://www.medrxiv.org/content/10.1101/2021.06.15.21258669v1.full.pdf

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibodies to mRNA vaccination
In a study published in the medRxiv* preprint server, researchers studied the immune response data to BNT162b2 after two doses. Participants were vaccinated with two doses of the BNT162b2 vaccine, 21 days apart. Immune response was tested about a week or two after the second dose. Participants also included some patients who were vaccinated after being naturally infected with SARS-CoV-2.
The team tested serum samples for IgG binding antibodies to the virus nucleocapsid protein and the spike protein. Of the 871 participants, 3.7% had IgG antibodies against the nucleocapsid protein and 99.7% had IgG antibodies to the spike protein receptor-binding domain (RBD).
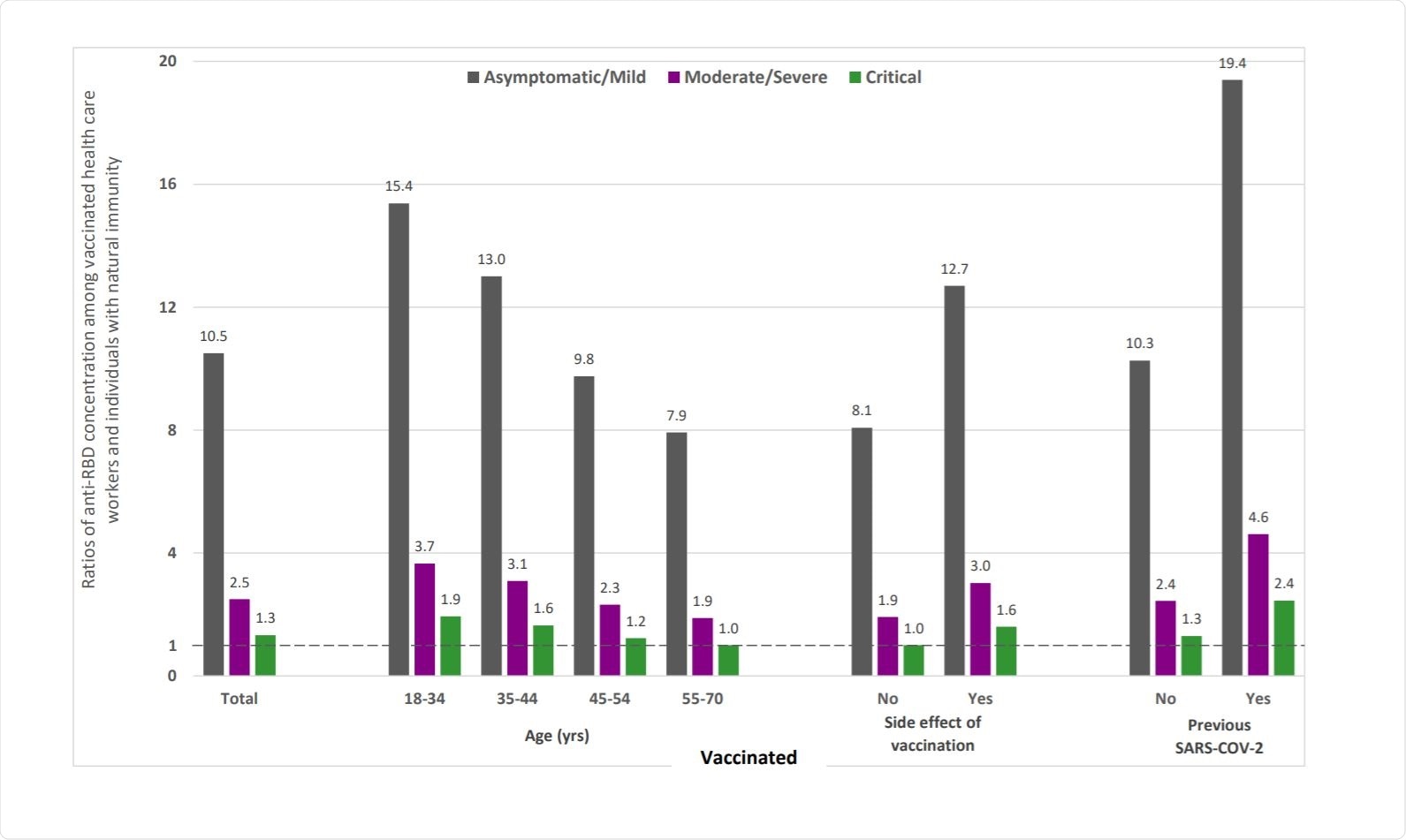
The researchers also found correlations between RBD antibodies and gender, age, and previous SARS-CoV-2 infection, with females having a higher concentration of antibodies than males. Elderly participants above 55 years had lower levels of antibodies than vaccinated people 18 to 34 years old. Those who had side effects also had higher levels of antibodies as did people who had had COVID-19 before.
Comparing antibodies to natural infection
The team also compared the data with people who only had natural SARS-CoV-2 infection. Among the people with only natural infection, 88.3% had antibodies against the nucleocapsid protein and 90.6% had anti-RBD antibodies.
The anti-RBD levels increased with disease severity, with critically ill patients having almost eight times the antibody concentrations as people with mild or moderate infection.
Comparing the antibody levels between those who had a natural infection and those who were vaccinated, the authors found that the ratio of anti-RBD levels in vaccinated individuals versus those with natural infection varied widely, from being a 1.0 to 19.4.
The results indicate that the BNT162b2 vaccine is highly immunogenic and elicits more antibodies than in response to natural infection. Real-world studies in countries like Qatar where the prevalence of the virus variants B.1.1.7 and B.1.351 is high showed 89% and 75% effectiveness against them, suggesting the mRNA vaccines can also protect against some virus variants.
The use of antibodies to understand protection against SARS-CoV-2 may provide useful tools to understand vaccine efficacy and effectiveness over time and against different virus variants, doing away with the need for doing expensive clinical trials.
The authors are continuing to study vaccinated and people infected naturally to understand the immune response, which will help with understanding how the virus infects and spreads as well as vaccine effectiveness.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources