Often patients suffering from COVID-19 (coronavirus disease) show striking immune dysregulation - causing increased morbidity and mortality. Mechanisms for adaptive immune disturbance, lymphopenia, and thromboinflammation in COVID-19 are still poorly understood.
Thromboinflammation plays a critical role through complement activation and cytokine release, platelet overactivity, apoptosis (thrombocytopathy), as well as coagulation abnormalities (coagulopathy). Vascular complications in COVID-patients links phosphatidylserine (PS) to thromboinflammation.
To understand the related aspects of COVID-19 in detail, researchers from Germany compared peripheral blood mononuclear cells (PBMC) between COVID-19 patients, healthy individuals, and those recovered from COVID-19. They found that the PS+ platelet-derived microparticles (PMP)-associated PBMC correlated with lymphopenia and disease severity, showing a higher correlation than the commonly used laboratory diagnostic markers such as IL-6 and D-Dimer and C-reactive protein (CRP). The results were published recently on the preprint server, bioRxiv*.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
"One of our most surprising findings was the high association of PBMC with PS+ PMPs and platelets over the disease course, shown with a novel PS-detecting method."
It is known that vascular complications may occur in COVID-19 patients where autoantibodies may target phosphatidylserine (PS)/prothrombin complexes. PS is a plasma membrane component that is actively retained by an ATP-requiring process at the inner membrane surface in living cells; it participates in the immune response. PS-enveloped foreign particles (such as microparticles or viruses) are released from cells to interact with extracellular proteins, including coagulation and complement systems.
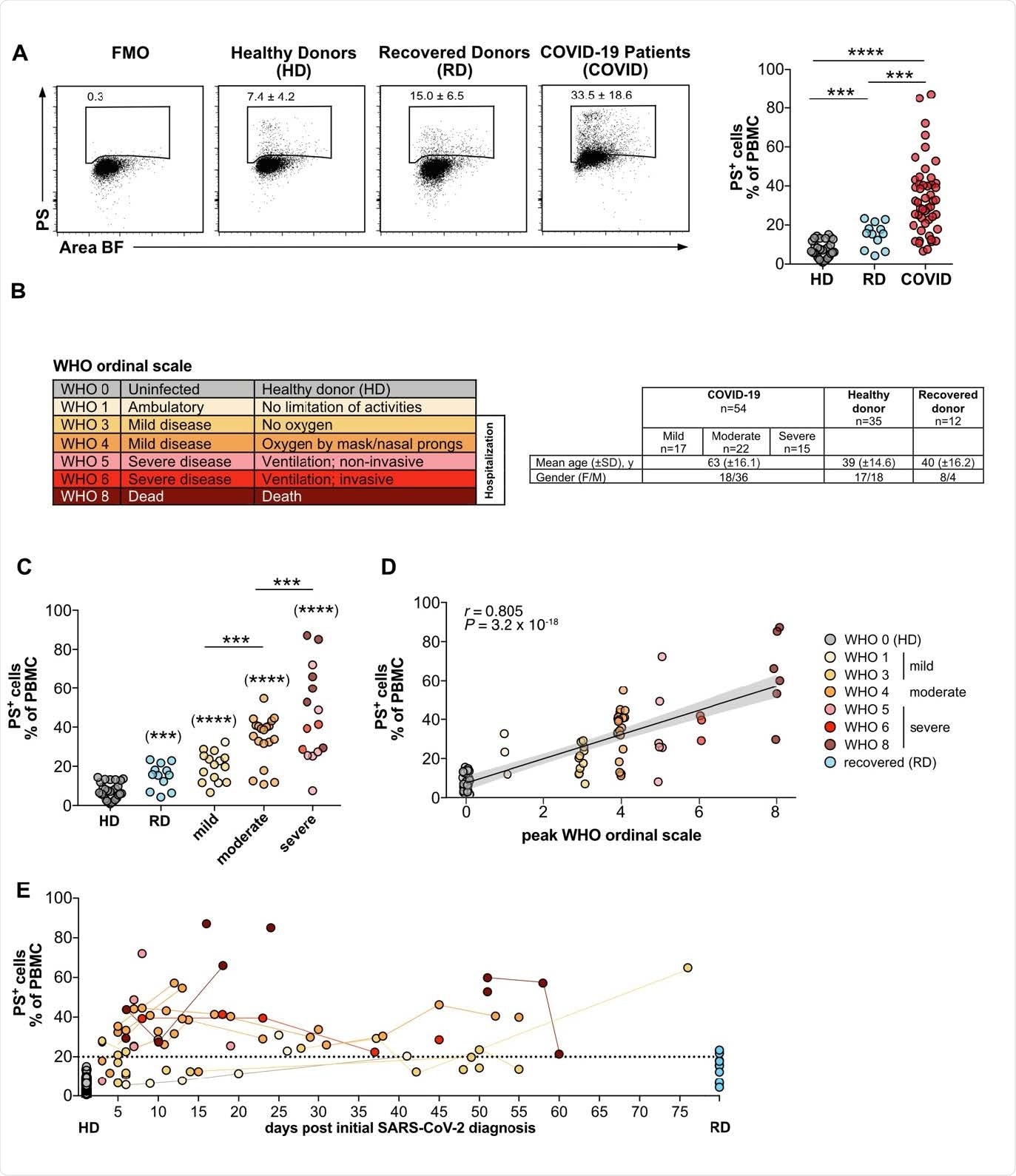
The frequencies of PS+ PBMC from COVID patients correlates with disease severity. (A) PBMC from COVID-19 patients (COVID), healthy donors (HD), and recovered donors (RD) were stained for PS and analyzed by flow cytometry. Numbers in dot plots correspond to the percentage of PS+ cells in the gate shown. The right-hand graph shows the summary of all percentages. (B) Overview of WHO ordinal scale and color code used. Table shows the number, age and gender of the different study groups. (C) Grouped analysis of the data from (A). (D) Same as (C), but plotted against the WHO ordinal scale (n = 38-79) (B). PS+ PBMCs correlate with the severity of the disease. The plot shows the Spearman correlation test and linear regression line with 95% confidence interval shading (A, C, D: HD, n = 30; RD, n = 12; COVID, n = 49). (E) Analysis of PS+ PBMC as shown in (A) plotted against days after initial SARS-CoV-2 diagnosis. Lines connect the same donors. Significance was determined by Mann-Whitney test: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Asterisks in brackets show statistically significant differences as compared to HD.
Once activated, the platelet-derived microparticles (PMPs) are released, which cause thrombin formation, coagulation, activation of the complement system, and inflammation in a PS-dependent manner. COVID-19 severity is associated with immune abnormalities such as increased inflammatory cytokines, immune cell exhaustion, and general lymphopenia.
Hyperactivated platelets, elevated levels of circulating PMPs, and an increased risk of thromboembolic complications - related to PS-associated immune mechanisms - contribute to COVID-19 disease severity and death.
"Since PMPs constitute the lion's share of CD41+PS+ particles on T cells of COVID-19 patients and PS may be responsible for the coagulation and inflammatory effects mentioned above, our findings are an unexpected result with high clinical relevance."
For this study, the researchers collected PBMC of 54 patients from the COVID-19 Registry of the LMU Munich (CORKUM) and 35 healthy and 12 recovered donors between April 2020 and February 2021. Using image flow cytometry (IFC) along with image analysis by deep learning algorithms using highly sensitive reagents specific for PS, the researchers identified the abnormally high numbers of PS+ PBMC in blood samples from COVID-19 patients.
The study significantly identified the severity of COVID-19 disease correlating with high significance with the frequencies of PS+ PBMC of COVID-19 patients. Moreover, they also reported that the elevated frequencies of PS+PBMC over time, for up to 10 weeks in some patients - indicating that the presence of PS+PBMC in COVID-19 patients might be long-lasting.
Also, they observed that in recovering patients, the PS+PBMC returned to the levels of healthy controls. "The number of PS+ PBMC in the blood of COVID-19 patients represents a new parameter that correlates strongly with disease severity," summarized the researchers.
"As PS+ PMPs remained associated with PBMC several weeks after the initial SARS-CoV2-diagnosis, they might sustain the adverse inflammatory and prothrombotic effects over a long time and contribute to the complex clinical picture of thromboinflammation."
To test if the PS+PBMC were apoptotic cells in severe COVID-19 patients, the researchers analyzed PBMC in COVID-19 patients are associated with PS+EVs (extracellular vesicles). They found that the PMBCs in COVID-19 patients are associated with PS+EVs.
When they investigated whether the PS+EVs would selectively associate with specific PBMC subpopulations, the researchers noted that the frequency of PS+ EV+ CD8+ T cells best reflected the severity of COVID-19 disease. In addition, the researchers reported that although PS is a marker for dying cells, nearly all the PS+cells were living cells associated with PS+ CD41+ PMPs or larger PS+ CD41+ platelet fragments.
The researchers discussed the underlying mechanism, showing that PS can trigger the blood coagulation cascade, the complement system, inflammation and reside on activated immune cells. "Therefore, PS may serve as a beacon to attract thromboinflammatory processes toward lymphocytes and cause immune dysfunction in COVID-19," the researchers concluded.
"Using novel PS-specific reagents based on lactadherin, we found few but statistically significantly increased amounts of dying cells in the 20 blood of COVID-19 patients with mild (WHO 1-3) and moderate disease (WHO 4)."
Because the degree of the association of PBMC with PS+PMPs and platelets correlated more strongly with COVID-19 severity than the established laboratory indices (such as lymphopenia, IL-6, D-Dimer, fibrinogen, and others) measured simultaneously, the researchers suggested a novel biomarker for disease severity - PS.
This study reveals an extensive association of PS+ PMPs with lymphocytes as a novel marker to classify COVID-19 disease severity and a potentially relevant contributor to thromboinflammation and lymphocyte dysfunction.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Binding of phosphatidylserine-positive microparticles by PBMCs classifies disease severity in COVID-19 patients, Lisa Rausch, Konstantin Lutz, Martina Schifferer, Elena Winheim, Rudi Gruber, Linus Rinke, Johannes Hellmuth, Clemens Scheerer, Maximilian Muenchhoff, Christopher Mandel, Michael Bergwelt-Baildon, Mikael Simons, Tobias Straub, Anne B Krug, Jan Kranich, Thomas Brocker, bioRxiv, 2021.06.18.448935; doi: https://doi.org/10.1101/2021.06.18.448935, https://www.biorxiv.org/content/10.1101/2021.06.18.448935v1
- Peer reviewed and published scientific report.
Rausch, Lisa, Konstantin Lutz, Martina Schifferer, Elena Winheim, Rudi Gruber, Elina F. Oesterhaus, Linus Rinke, et al. 2021. “Binding of Phosphatidylserine‐Positive Microparticles by PBMCs Classifies Disease Severity in COVID‐19 Patients.” Journal of Extracellular Vesicles 10 (14). https://doi.org/10.1002/jev2.12173. https://onlinelibrary.wiley.com/doi/full/10.1002/jev2.12173.