Researchers in the United States have conducted a study showing that the humoral (antibody) immunity induced by vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19) – varies significantly among immunocompromised individuals.
The findings come from an interim analysis of an ongoing observational, prospective cohort analysis called the COVID-19 Vaccination in the Immunocompromised Study (CoVICS), which began on April 14th, 2021.
Ghady Haidar and colleagues report that the presence of antibodies (seropositivity) against SARS-CoV-2 was significantly lower among certain groups of immunocompromised individuals compared with among healthy vaccinees.
Seropositivity was significantly lower among immunocompromised individuals who had received solid organ transplants (SOT) and those with hematologic malignancies.
By contrast, among immunocompromised individuals with solid tumors or autoimmune conditions, seropositivity approached that observed among healthy individuals.
The study also found that more than 90% of patients with human immunodeficiency virus (HIV) were seropositive.
The researchers from the University of Pittsburgh School of Medicine and the University of Pittsburgh Medical Center in Pennsylvania say the findings demonstrate how the humoral response to COVID-19 vaccines significantly varies, depending on the underlying immunosuppressive condition.
There is an urgent need to optimize and individualize approaches to COVID-19 prevention among these patients, they add.
A pre-print version of the research paper is available on the medRxiv* sever, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Immunocompromised individuals have been excluded from vaccine efficacy trials
Immunocompromised patients are at an increased risk for severe and protracted illness following infection with SARS-CoV-2.
While these individuals should therefore be prioritized for COVID-19 vaccination, the presence of confounding comorbidities has meant their exclusion from clinical trials evaluating the immunogenicity and efficacy of vaccines.
Not surprisingly, recent studies have shown that vaccination elicits antibody responses that fall well below the 100% response rates observed among healthy individuals.
“Despite these emerging data, several unknowns persist, including the degree of the antibody response in seropositive immunocompromised patients, and whether antibodies from immunocompromised patients are capable of neutralizing SARS-CoV-2,” writes Haidar and the team.
What did the researchers do?
The researchers conducted an interim analysis of the ongoing CoVICS study. The analysis involved 107 HCWs and 489 immunocompromised patients who had been fully immunized with the Moderna, Pfizer-BioNTech or Johnson & Johnson vaccine.
Fourteen days following completion of vaccination, serum samples were collected and tested for the presence of immunoglobulin G (IgG) against the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein.
The spike protein mediates the initial stage of infection when its RBD binds to the host cell receptor angiotensin-converting enzyme 2 (ACE2). The spike RBD is a major target of binding and neutralizing antibodies following natural infection or vaccination.
The researchers also selected a subset of participants who had their blood tested in pseudovirus neutralization assays.
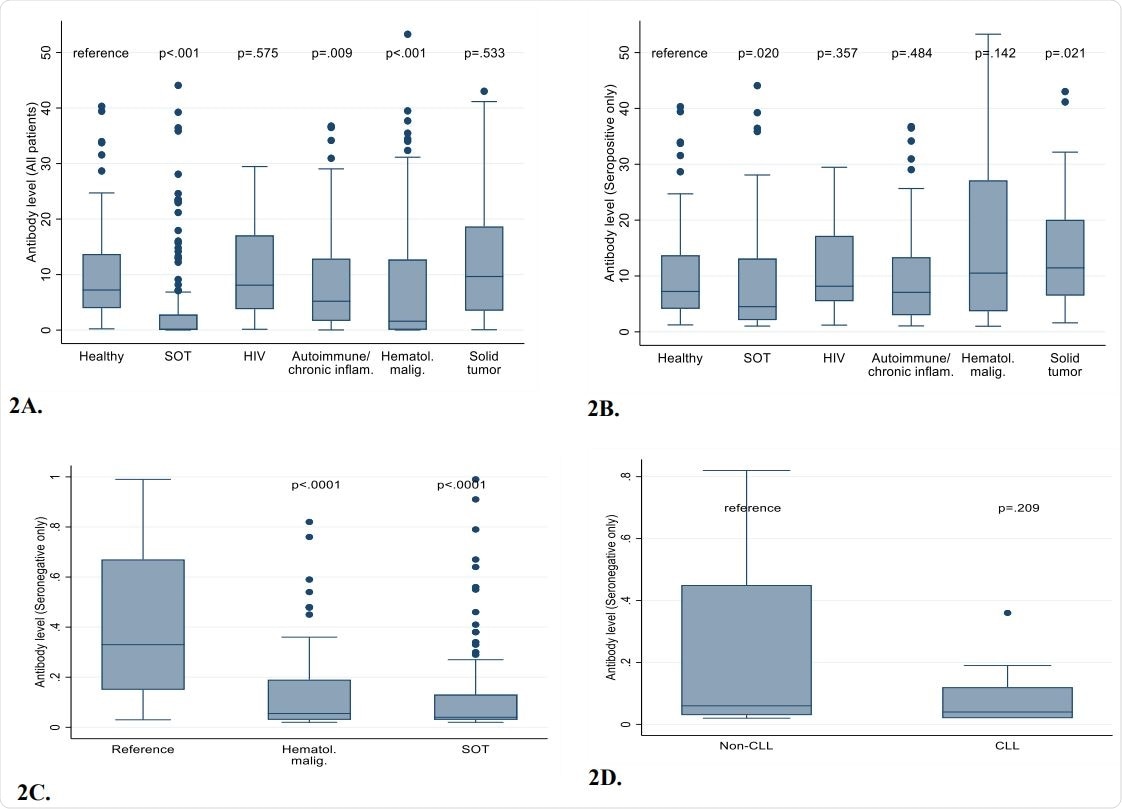
All antibody levels (seropositive and seronegative patients) in healthy healthcare workers and immunocompromised patients. Figure 2B. Comparisons of antibody levels among only patients with positive results. Figure 2C. Comparison of antibody levels among only patients with negative results, demonstrating that near absence of antibodies in many SOT recipients and patients with hematological malignancies. Figure 2D. Comparison of antibody levels CLL vs non-CLL hematological malignancy patients with negative results.
What did the study find?
Among the immunocompromised patients, 183 (37.4%) had received a solid organ transplant (SOT), 160 (32.7%) had an autoimmune condition, 75 (15.3%) had a hematologic malignancy, 37 (7.6%) had HIV and 34 (7.0%) had solid tumors.
Compared with HCWs, seropositivity was significantly lower among immunocompromised patients with SOT (37.2%) or hematologic malignancies (54.7%), than among HCWs (98.1%).
Among the SOT recipients, lung transplant recipients had the lowest seropositivity (22.2%), while liver transplant recipients had the highest seropositivity (60.6%).
Seropositivity was also lower among immunocompromised individuals with solid tumors (82.4%) or autoimmune conditions (83.8%), but was closer to the that observed for HCWs.
Importantly, well-controlled patients with HIV mounted antibody responses that were almost identical to those of healthy HCWs.
“Although it is extremely encouraging that 94% of participants with HIV responded to the vaccines, this group of patients continues to be a marginalized group with poor access to vaccination, and outreach efforts should focus on increasing awareness of vaccination in these patients,” warns Haidar and colleagues.
The study also found that SARS-CoV-2 neutralization titers were generally strongly correlated with anti- RBD IgG levels. However, more extensive studies will be needed to fully evaluate whether subsets of immunocompromised patients fail to neutralize the virus, adds the team.
What did the authors conclude?
“Taken together, our findings demonstrate the heterogeneity of the humoral immune response to COVID-19 vaccines based on underlying immunosuppressive condition and highlight an urgent need to optimize and individualize COVID-19 prevention in these patients,” says Haidar and colleagues.
The researchers say the findings also have important implications for public health guidance, particularly given that revised guidelines from the Centers for Disease Control and Prevention permit vaccinated individuals to abandon masking and social distancing in most settings.
“Future studies are warranted to determine assessment of cellular immunity, longitudinal measurement of immune responses, and the safety and efficacy of revaccination,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Haidar G, et al. Immunogenicity of COVID-19 Vaccination in Immunocompromised Patients: An Observational, Prospective Cohort Study Interim Analysis. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.28.21259576, https://www.medrxiv.org/content/10.1101/2021.06.28.21259576v1
- Peer reviewed and published scientific report.
Haidar, Ghady, Mounzer Agha, Andrew Bilderback, Amy Lukanski, Kelsey Linstrum, Rachel Troyan, Scott Rothenberger, et al. 2022. “Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses across a Broad Spectrum of Immunocompromising Conditions: The COVID-19 Vaccination in the Immunocompromised Study (COVICS).” Clinical Infectious Diseases 75 (1): e630–44. https://doi.org/10.1093/cid/ciac103. https://academic.oup.com/cid/article/75/1/e630/6530582.