With the catastrophic impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on populations worldwide, there has been an urgent need to develop effective vaccines and drugs that can target this virus. As of July 5, 2021, almost 185 million people have been infected by SARS-CoV-2, with almost 4 million people succumbing to the coronavirus disease 2019 (COVID-19).
Despite the global roll-out of several vaccines, there remains a lack of affordable drugs that are effective against SARS-CoV-2. The research into antiviral therapeutics has been especially important for treating patients who experience severe symptoms of COVID-19. Additionally, the emergence of new SARS-CoV-2 variants of concern (VOCs) has further increased the need to develop and administer effective therapeutics.
 Study: Large-scale screening discovers clofoctol as an inhibitor of SARS-CoV-2 replication that reduces COVID-19-like pathology. Image Credit: unoL / Shutterstock.com
Study: Large-scale screening discovers clofoctol as an inhibitor of SARS-CoV-2 replication that reduces COVID-19-like pathology. Image Credit: unoL / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Repurposing available drugs
The aim of repurposing clinically evaluated drugs that are already available for their use against SARS-CoV-2 would allow effective drugs to reach patients much more quickly as compared to novel drugs that have not already undergone these processes.
In a recent study published on the pre-print server bioRxiv* a group of French researchers aimed to reduce the regulatory preclinical development steps that potential candidates are normally subjected to. To this end, the researchers investigated several existing drug compounds for their potential antiviral activity against SARS-CoV-2,
Evaluating antiviral activity of available drugs
In their work, the researchers developed a high-content screen (HCS) using a drug library called Apteeus (TEELibrary®), which included a comprehensive collection of 1,942 approved drugs. The HCS method was used to screen and identify molecules that exhibit antiviral activity against SARS-CoV-2.
The drug screen was performed on an African green monkey kidney cell line known as Vero-81. Vero-81 cells, which were chosen for this experiment due to their known susceptibility to SARS-CoV-2, were used to assess the measurable outcomes of the antiviral activity of the chosen drug compounds. Notably, a majority of the compounds that were assessed in this drug screen were based on known basic molecules like chloroquine (CQ), amodiaquine, fluphenazine, trifluoperazine, and triflupromazine.
SARS-CoV-2, along with other coronaviruses, can utilize two different entry points to gain access to the host cell. This can include entering the cell through endocytosis and releasing the viral genome into the cytosol after the fusion of the envelope with the endosomal membrane. SARS-CoV-2 can also enter host cells by fusing its viral envelope directly with the plasma membrane of host cell. The second method is stimulated by the cell surface protease TMPRSS2; however, this is enzyme not expressed in the Vero-81 cells.
CQ has been previously identified to exhibit antiviral activity against SARS-CoV-2 in Vero-18 cells by blocking the endocytic entry route of the virus into host cells. However, when tested on Vero-81 cells ectopically expressing TMPRSS2 (Vero-81-TMPRSS2), CQ was not successful in inhibiting the entry of SARS-CoV-2 into the cells. These findings, therefore, supported testing the drug compounds on both Vero-81 and Vero-81-TMPRSS2 cells in order to eliminate compounds that only blocked one route of SARS-CoV-2 entry.
Of all the compounds that were initially screened in this study, only three were found to exhibit a dose-dependent antiviral effect against SARS-CoV-2 in Vero-81 cells, both in the presence and absence of TMPRSS2. These compounds included perphenazine, nitazoxaine, and clofoctol; of which clofoctol was chosen to further study for its anti-SARS-CoV-2 properties.
Clofoctol
Clofoctol is an antibacterial drug that was first developed in the 1970s and has been shown to be effective in the treatment of Streptococcus pneumoniae, which is the leading global cause of bacterial pneumonia worldwide, as well as Staphyloccus aureus. Clofoctol works by inhibiting bacterial cell wall synthesis as well as inducing membrane permeability. It has also recently been found to inhibit protein translation and impair tumor growth.
The researchers of the current study also found that clofoctol can inhibit SARS-CoV-2 by blocking the translation of viral ribonucleic acid (RNA), which is critical for the spread of the virus. This may be due to the activation of the unfolded protein response (UPR) pathways, as there have been reports of this drug compound being able to induce endoplasmic reticulum stress and activate all three UPR pathways. These pathways include inositol requiring enzyme 1 (IRE1), the double-stranded RNA-activated PK-like ER kinase (PERK), and the activating transcription factor 6 (ATF6).
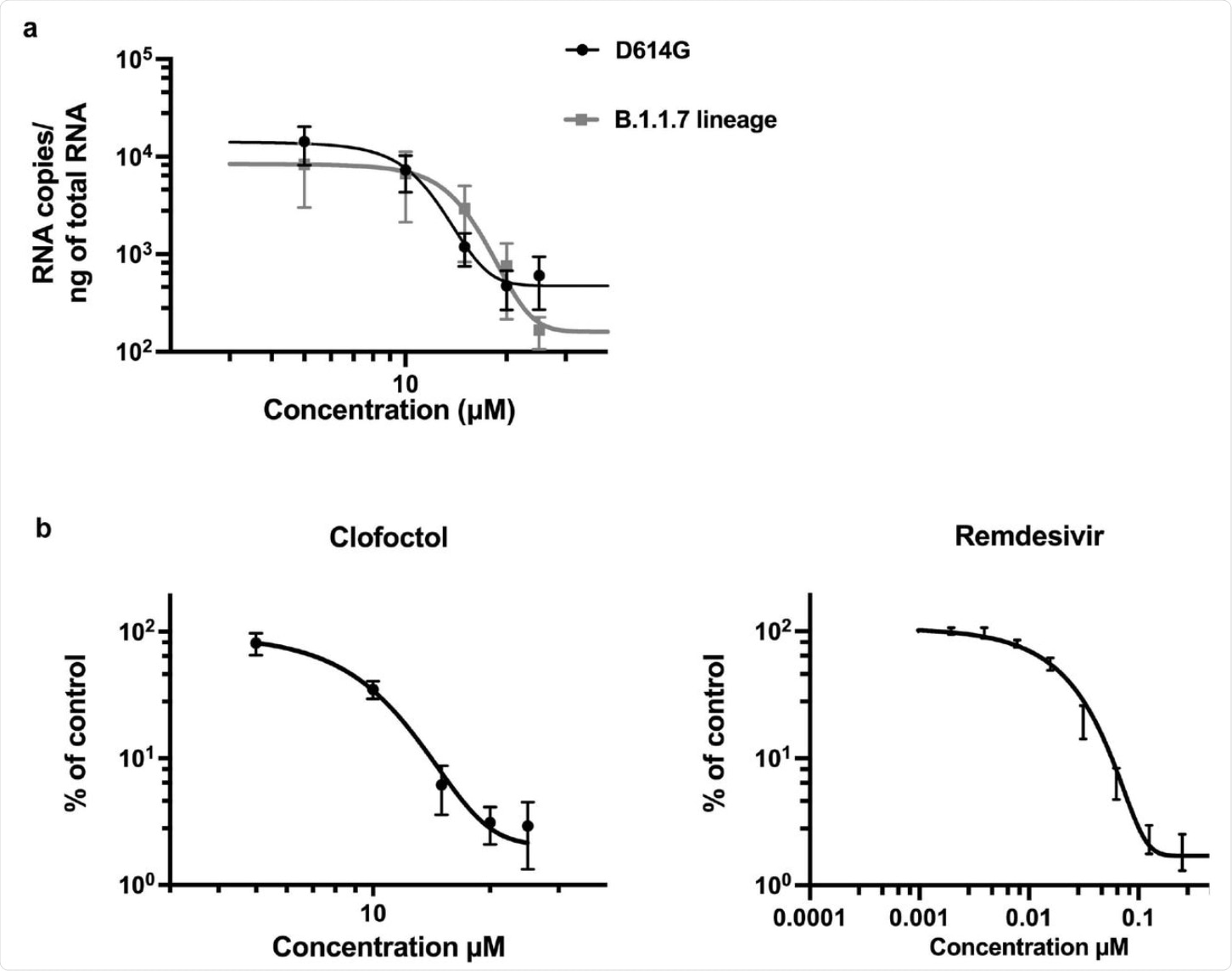 Clofoctol inhibits other SARS-CoV-2 variants as well as HCoV-229E. a, Vero-81 cells were infected either with SARS-CoV-2 of lineage B1 containing the D614G mutation (SARS-CoV-2/human/FRA/Lille_Vero-TMPRSS2/2020) or with SARS-CoV-2 of lineage B1.1.7 (GISAID accession number EPI_ISL_1653931). Viral genomes were quantified by RT-qPCR. Results are normalized by the amount of total RNA and represent the average of three independent experiments performed in duplicates. b, Huh-7 cells were infected with HCoV-229Ė-Rluc in presence of different concentrations of clofoctol or remdesivir. At 7h post-infection, cells were lysed and luciferase activities were quantified. Results are presented as the percentages of the control and represent an average of three independent experiments performed in triplicates. Errors bars represent the standard error of the mean (SEM).
Clofoctol inhibits other SARS-CoV-2 variants as well as HCoV-229E. a, Vero-81 cells were infected either with SARS-CoV-2 of lineage B1 containing the D614G mutation (SARS-CoV-2/human/FRA/Lille_Vero-TMPRSS2/2020) or with SARS-CoV-2 of lineage B1.1.7 (GISAID accession number EPI_ISL_1653931). Viral genomes were quantified by RT-qPCR. Results are normalized by the amount of total RNA and represent the average of three independent experiments performed in duplicates. b, Huh-7 cells were infected with HCoV-229Ė-Rluc in presence of different concentrations of clofoctol or remdesivir. At 7h post-infection, cells were lysed and luciferase activities were quantified. Results are presented as the percentages of the control and represent an average of three independent experiments performed in triplicates. Errors bars represent the standard error of the mean (SEM).
The interference of these UPR pathways has been reported to block the replication of viruses such as SARS-CoV-2. Further studies must be conducted to confirm whether clofoctol-induced inhibition of SARS-CoV-2 through translation is associated with activating the UPR pathways.
Conclusion
The team of researchers in this study demonstrated that clofoctol exhibits antiviral activity against SARS-CoV-2 in vitro. Additionally, in vivo data conducted in this study found that clofoctol decreased the viral load of SARS-CoV-2 in the lungs of transgenic C57BL-6 mice expressing the human angiotensin-converting enzyme 2 (ACE2) receptor and also significantly reduced pulmonary inflammation.
Taken together, the findings of this study led the researchers to recommend clofoctol as a strong candidate for the treatment of SARS-CoV-2. A phase 2/3 placebo trial is currently being planned to further validate this drug for its use against COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Belouzard, S., Machelart, A., Sencio, V., et al. (2021). Large scale screening discovers clofoctol as an inhibitor of SARS-CoV-2 replication that reduces COVID-19-like pathology. bioRxiv. doi:10.1101/2021.06.30.450483. https://www.biorxiv.org/content/10.1101/2021.06.30.450483v1
- Peer reviewed and published scientific report.
Belouzard, Sandrine, Arnaud Machelart, Valentin Sencio, Thibaut Vausselin, Eik Hoffmann, Nathalie Deboosere, Yves Rouillé, et al. 2022. “Clofoctol Inhibits SARS-CoV-2 Replication and Reduces Lung Pathology in Mice.” Edited by Vineet D. Menachery. PLOS Pathogens 18 (5): e1010498. https://doi.org/10.1371/journal.ppat.1010498. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010498.