Malaria is a life-threatening mosquito-borne disease that is caused by one of four parasites including the Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae.
In 2019 alone, over 229 million cases of malaria were reported globally, with about 409,000 deaths due to this disease. Although several drug and anti-vector measures have been developed to mitigate the morbidity and mortality associated with malaria, the progress in combatting this disease has stalled.
 Study: Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Image Credit: Jarun Ontakrai / Shtuterstock.com
Study: Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Image Credit: Jarun Ontakrai / Shtuterstock.com
Malaria vaccine candidates
The Mosquirix (RTS,S) and Sanaria Plasmodium falciparum sporozoites (PfSPZ) vaccines are considered the most advanced candidates for conferring sterile protective immunity against malaria. The RTS,S vaccine targets the circumsporozoite protein (CSP) of PfSPZ and has demonstrated its ability to provide partial protection against clinical malaria.
Comparatively, the Sanaria PfSPZ vaccine is composed of radiation-attenuated PfSPZ offers significant protection against both homologous and heterozygous against controlled human malaria infection (CHMI).
A team of researchers recently optimized regimens for chemoprophylaxis vaccination (CVac), wherein aseptic, purified, cryopreserved, and infectious PfSPZ were inoculated under prophylactic cover and mixed with pyrimethamine (PYR) and chloroquine (CQ). The vaccine with pyrimethamine is called Sanaria PfSPZ-CVac(PYR), while the vaccine with chloroquine is called PfSPZ-CVac(CQ).
Vaccine efficacy
In the first clinical trial (ID: NCT02511054), the researchers compared three monthly doses of the PfSPZ-CVac at a low dose combined with weekly treatments of 500 mg of CQ with three monthly doses of the PfSPZ-CVac at a low dose combined with 50 mg of PYR on days 2 and 3, along with weekly CQ treatment.
In this study, the researchers found that the incorporation of PYR successfully killed liver-stage parasites in all recipients. Notably, four out of five of the recipients who were on the former treatment protocol were protected against CHMI for 3 months after the last dose, whereas only two out of the nine of the recipients in the latter group were protected for the same time period.
In a second dose-escalating study (ID: NCT03083847), the researchers found that a four-fold increase in the dose of PfSPZ-CVac(PYR) transformed a minimal vaccine efficacy to a high-level vaccine efficacy of 87.5% and 77.8% against homologous and heterologous CHMI, respectively, three months after the third vaccination. Notably, these higher doses of the vaccines were found to be safe and well-tolerated among most participants.
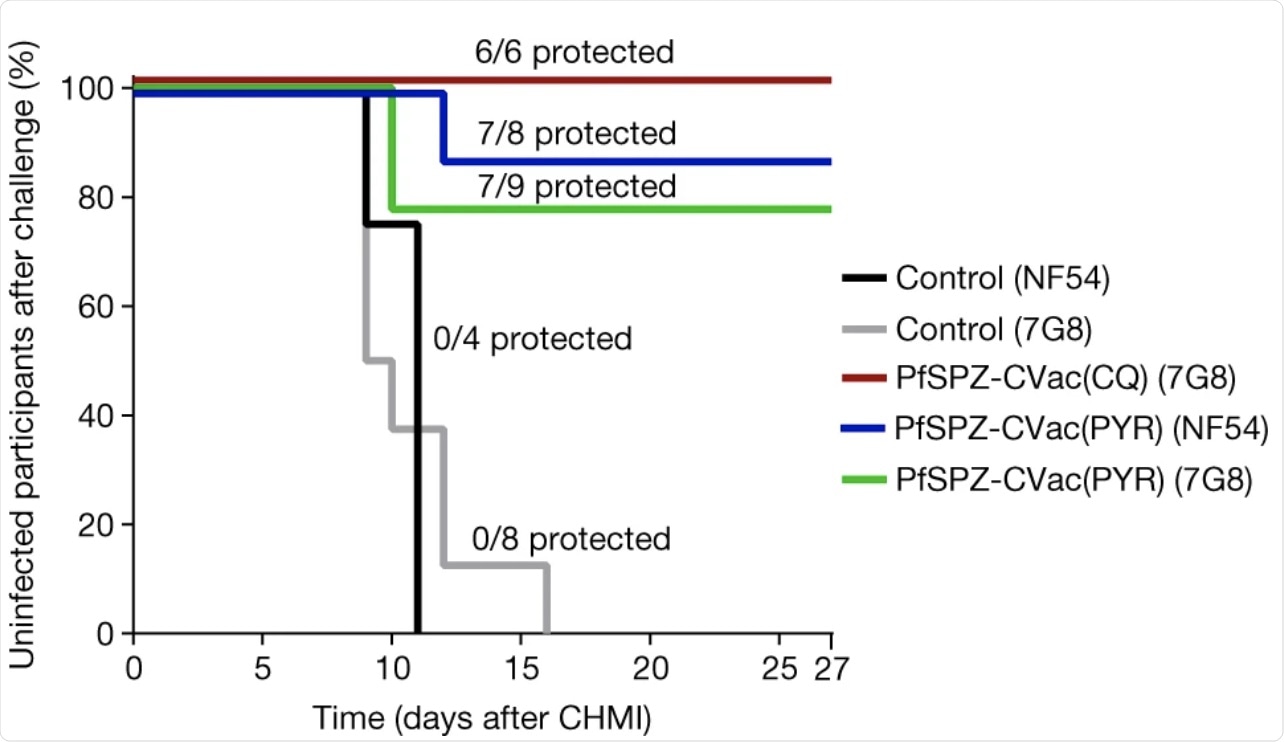 Survival curves display the percentage of study participants who remained protected throughout 27 days of follow-up after inoculation with a high dose of PfSPZ (2 × 105). Vaccine efficacy compared the vaccinated to unvaccinated groups for the time to first detectable parasitaemia after inoculation using a log-rank test. In the PfSPZ-CVac(PYR) group, 7/8 participants (vaccine efficacy = 87.5%, P = 0.003; 95% confidence interval, 42.5–100%) were protected from homologous CHMI and 7/9 participants (vaccine efficacy = 78%, P = 0.001; 95% confidence interval, 39.8–100%) from heterologous CHMI. In the PfSPZ-CVac(CQ) group, 6/6 participants (vaccine efficacy = 100%, P = 0.001; 95% confidence interval, 54.1–100%) were protected from heterologous CHMI.
Survival curves display the percentage of study participants who remained protected throughout 27 days of follow-up after inoculation with a high dose of PfSPZ (2 × 105). Vaccine efficacy compared the vaccinated to unvaccinated groups for the time to first detectable parasitaemia after inoculation using a log-rank test. In the PfSPZ-CVac(PYR) group, 7/8 participants (vaccine efficacy = 87.5%, P = 0.003; 95% confidence interval, 42.5–100%) were protected from homologous CHMI and 7/9 participants (vaccine efficacy = 78%, P = 0.001; 95% confidence interval, 39.8–100%) from heterologous CHMI. In the PfSPZ-CVac(CQ) group, 6/6 participants (vaccine efficacy = 100%, P = 0.001; 95% confidence interval, 54.1–100%) were protected from heterologous CHMI.
With the chloroquine combination, a 100% efficacy rate was reported in those who received the higher PfSPZ dosage, thereby indicating that this vaccine conferred protection against the heterologous challenge. This trial also revealed that the high levels of cross-strain protection lasted at least three months for both the higher doses.
“One hundred percent protection for three months against heterologous variant parasites is unprecedented for any malaria vaccine in development.”
This showed that the CVac could be a novel and promising approach for the immunization of travelers to and residents living in malaria-endemic regions.
“This trial will be the first step in establishing a new regimen for the PfSPZ-CVac approach, exposure to liver-stage parasites without subsequent blood-stage parasites, assessing protection against homologous CHMI.”
In future trials, the two-drug regimen can be assessed for protection against heterologous Pf infection and longevity of protection. The results of the study will contribute to understanding the targets and mechanisms of immunity against Pf malaria infection.”
Fight against malaria
At present, the RTS,S vaccine has been found to provide partial protection in young children against malaria. However, in late-stage clinical trials, RTS,S was found to only prevent 39% of malaria cases among children between 5 and 17 years old. Another promising malaria vaccine candidate is known as R21, which also targets the CSP of P. falciparum, has shown 77% efficacy among children.
A Phase 2 clinical trial of CVac is now underway in Mali, which is a malaria-endemic country. If the approach is effective and successful, it can help decrease the increasing number of malaria cases worldwide. Since there remains no vaccine against malaria that is used worldwide, the CVac approach may provide hope for people residing in malaria-endemic areas.