Researchers in the United States and India have presented the first known evidence that the rollout of coronavirus disease 2019 (COVID-19) vaccination is restricting the evolutionary and immune escape pathways accessible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Venky Soundararajan from nference Labs, in Cambridge, Massachusetts and colleagues found that the diversity of SARS-CoV-2 lineages is declining at the country-level as rates of mass COVID-19 vaccination increase.
The researchers also found that compared with unvaccinated COVID-19 patients, vaccinated individuals who developed breakthrough SARS-CoV-2 infection harbored viruses with significantly lower diversity in the B cell epitopes that are leveraged following vaccination.
“This study demonstrates that mass vaccination may serve as an antigenic impediment to the evolution of fitter and more transmissive SARS-CoV-2 variants, emphasizing the urgent need to stem vaccine hesitancy as a key step to mitigate the global burden of COVID-19,” writes the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Understanding SARS-CoV-2 evolution is imperative to combating the pandemic
The host immune response following SARS-CoV-2 infection is a key selective pressure that influences the emergence of novel viral strains.
“Understanding the longitudinal trends of SARS-CoV-2 evolution and mapping the mutational landscape of the antigen is imperative to comprehensively combat the ongoing pandemic and future outbreaks,” says Soundararajan and colleagues.
The rapid development of COVID-19 vaccines and the mass rollout of vaccination across many countries has led to more than 800 million individuals now being fully immunized globally.
Such accelerated immunization of a large proportion of the population at the height of the ongoing pandemic could significantly increase evolutionary pressure on the SARS-CoV-2 virus, warn the researchers.
“However, to date, there has been no comprehensive study on the impact of the global vaccination efforts on SARS-CoV-2 evolution,” they write.
Availability of globally shared data provides a “timely opportunity”
Since the COVID-19 outbreak first began in late December 2019, global data-sharing efforts resulted in more than 1.8 million SARS-CoV-2 genomes from 183 countries and territories being deposited in the Global Initiative on Sharing Avian Influenza Data (GISAID) database by May 2021.
“The availability of genomic and immunological data provides a timely opportunity to systematically characterize the antigenic mutational landscape of SARS-CoV-2,” says Soundararajan and colleagues.
What did the researchers do?
The researchers conducted a longitudinal analysis of the SARS-CoV-2 genomes available in the GISAID database to capture vaccination-associated viral evolutionary patterns. They also performed viral genome sequencing for 23 vaccinated patients with breakthrough COVID-19 and 30 unvaccinated COVID-19 patients.
Analysis of the 1.8 million SARS-CoV-2 genomes revealed a total of 1,296 different viral lineages.
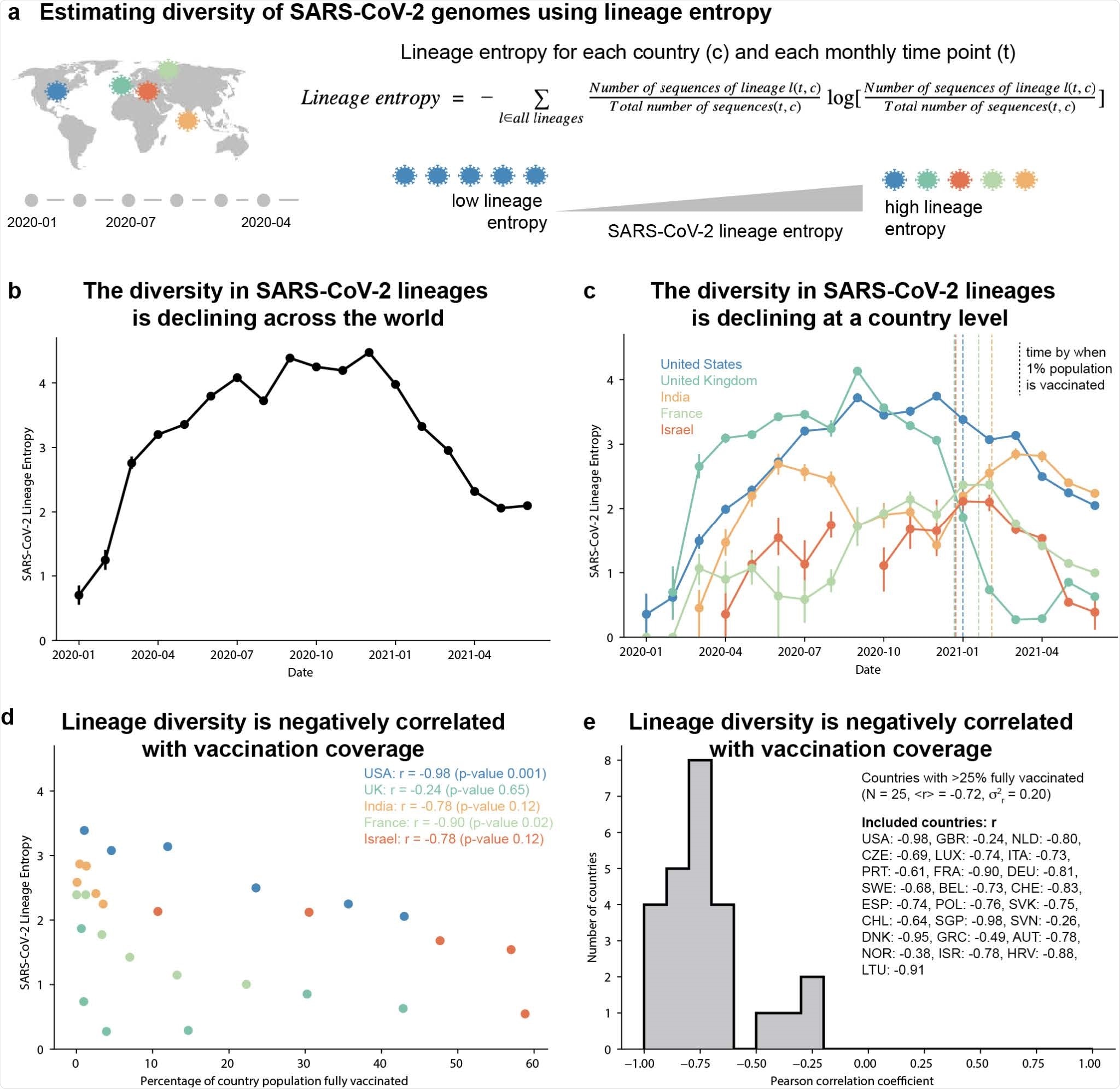
SARS-CoV-2 genomes show a global decline in sequence diversity coinciding with mass vaccination for COVID-19. (a) Schematic overview of estimating genomic diversity of SARS-CoV-2 (b-c) Diversity in SARS-CoV-2 lineages within the GISAID data, quantified using the entropy of the lineage probability distribution within 1-month time windows. Vertical dashed lines indicate the time at which countries reached a vaccine coverage of 1% of their total population. (d) Scatter plot showing the correlation between the country-level percentage of fully vaccinated individuals (from OWID21) and SARS-CoV-2 lineage entropy. (e) Distribution of the Pearson correlation coefficient between country-level percentage of fully vaccinated individuals and SARS-CoV-2 lineage entropy for all 25 countries with greater than 25% of their population fully vaccinated (as of June 26th, 2021) and at least 4-months with 100 or more sequences deposited to GISAID, after start of vaccination. The ISO 3166-1 alpha-3 code of included countries and their Pearson correlation are listed in the figure legend.
Strikingly, the team found that the diversity of these lineages has declined globally, with this decline appearing to coincide with the onset of mass vaccination rollout.
When the researchers analyzed the relationship between vaccination rates and lineage entropy in 25 countries where more than 25% of the population was fully vaccinated, they found that the decline in lineage diversity was indeed correlated with increased rates of mass vaccination.
Furthermore, the decline in lineage diversity was coupled with increased dominance of the B.1.1.7 (alpha), B.1.1.617 (delta) and P.1 (gamma) variants of concern, suggesting that these variants may be “fitter” SARS-CoV-2 lineages.
B cell epitopes had a higher mutational burden than T cell epitopes
Given that the COVID-19 vaccines leverage B-cell and T-cell epitopes, the team analyzed the prevalence of mutations in each known epitope residue.
This revealed a higher mutational burden in neutralizing B cell epitopes than in neutralizing T cell epitopes.
Furthermore, variants of concern contained more mutations in B-cell epitopes than non-variants of concern.
The team says the results suggest that the SARS-CoV-2 spike protein is presently undergoing strong B cell-driven selection pressure, with variants of concern exhibiting the most significant increases in B-cell epitope mutations.
The viral spike protein is the primary structure that SARS-CoV-2 uses to infect host cells and the main site harboring emergent mutations that confer the increased transmissibility and immune escape that has been observed for certain variants.
Indeed, the genomic sequencing of SARS-CoV-2 genomes from COVID-19 patients revealed that the unvaccinated individuals shared significantly more B-cell epitope mutational similarity with variants of concern than the vaccinated patients who developed a breakthrough infection.
What did the authors conclude?
“This study presents the first known evidence that COVID-19 vaccines are fundamentally restricting the evolutionary and antigenic escape pathways accessible to SARS-CoV-2,” says Soundararajan and colleagues.
The researchers say that while the analysis did not directly predict variant neo-epitopes, it highlights the importance of epitopes that are recurrently mutated during viral evolution in response to immune pressure.
“The societal benefit of mass vaccination may consequently go far beyond the widely reported mitigation of SARS-CoV-2 infection risk and amelioration of community transmission, to include the stemming of rampant viral evolution,” they conclude.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.