In the context of the coronavirus disease 2019 (COVID-19) pandemic, which is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the lack of effective and safe antivirals prompted many countries to approve the use of vaccines as the only way out of this situation. However, the antibody response following vaccination needs to be assessed to ensure that it is clinically effective in neutralizing the virus.
 Study:Initial SARS-CoV-2 vaccination response can predict booster response for BNT162b2 but not for AZD1222. Image Credit: felipe caparros / Shutterstock.com
Study:Initial SARS-CoV-2 vaccination response can predict booster response for BNT162b2 but not for AZD1222. Image Credit: felipe caparros / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The first COVID-19 vaccines that were developed included the messenger ribonucleic acid (mRNA) Pfizer and Moderna vaccines. The mRNA technology of these vaccines encodes for the SARS-CoV-2 spike (S) protein, which will ultimately be translated by the vaccine recipient’s cells.
By the end of 2020, both the Pfizer and Moderna vaccines, along with several other notable COVID-19 vaccines, such as the AstraZeneca adenovirus vector vaccine, have been approved for public use in many countries around the world.
In some centers, post-vaccination titers of antibodies elicited against the S protein are routinely measured. Post-vaccination titers typically vary between individuals because of the different analytical systems used at each center. The age and prior serological status of the vaccine recipient are also important factors that can determine post-vaccination titers. Additionally, the type of vaccine and the duration since the last dose, as well as priming and booster doses, also contribute significantly to the final serologic result.
There remains a limited amount of data on the correlation that exists between the antibody response to the first dose and the second dose. It appears likely that the type of vaccine will play a role in determining the type of immune response, especially since vector vaccines will induce antibodies against the vector virus as well as the SARS-CoV-2 S protein.
What did this study show?
The current report evaluates the ability to predict antibody levels after the booster dose of either the BNT162b2 (Pfizer/BioNTech) or AZD1222 (AstraZeneca) vaccine.
Antibody titers rose steadily from 3-12 weeks after the first AZD1222 dose from 13 BAU/mL to 56 BAU/mL.
The level of antibodies elicited by the prime dose of the BNT162b2 vaccine were found to predict the immune response to the booster dose; conversely, the AZD1222 vaccine did not show this predictability. This observation occurred in vaccine recipients of comparable age, approximately 42 years.
Before receiving the second dose of the vaccine, the antibody titers were 56 BAU/mL and 81 BAU/mL for the AZD1222 and BNT162b2 vaccine, respectively. These titers were measured at 11 weeks and 3 weeks after the first dose, respectively. At 21 days after the booster dose, antibody titers rose to approximately 1,000 and 2,000 BAU/mL for AZD1222 and BNT162b2, respectively.
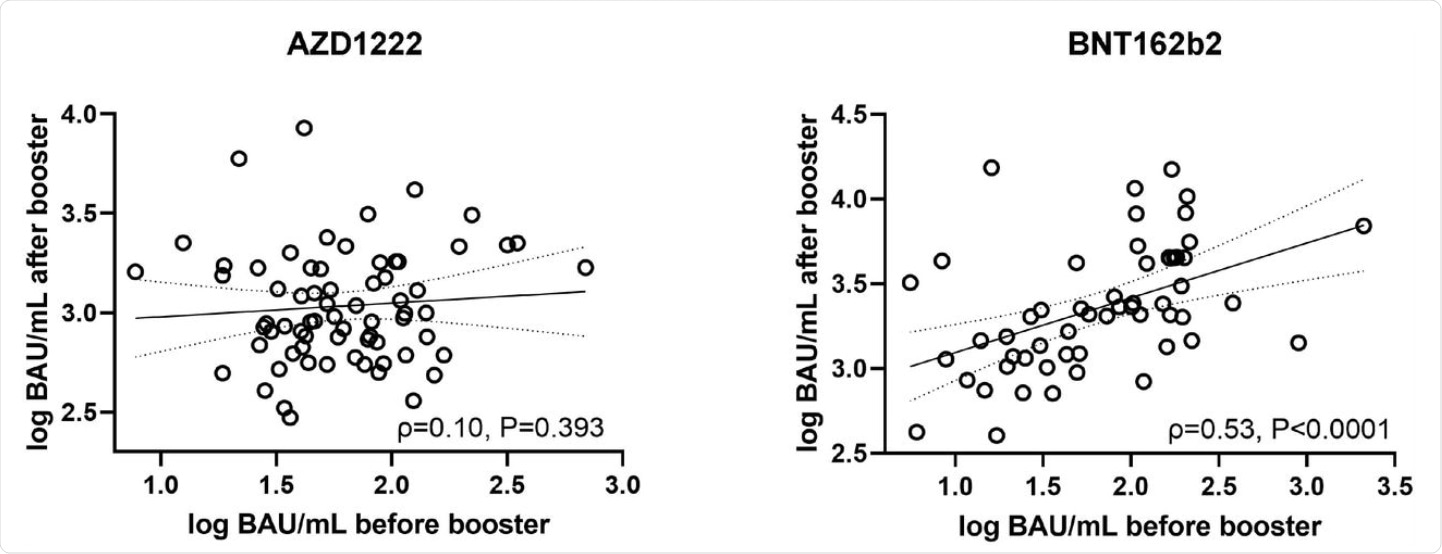 Correlation (according to Spearman) between pre- and post-booster antibody levels (Roche Elecsys S total antibody ECLIA) in AZD1222 (left) and BNT162b2 (right) vaccinees. BAU/mL… binding antibody units per milliliter.
Correlation (according to Spearman) between pre- and post-booster antibody levels (Roche Elecsys S total antibody ECLIA) in AZD1222 (left) and BNT162b2 (right) vaccinees. BAU/mL… binding antibody units per milliliter.
For the AZD1222 vaccine, age was not found to affect antibody levels after the priming dose; however, a weak association was observed after the second dose. In contrast, the antibody titers were higher in younger people after the first dose of the BNT162b2 vaccine; however, this association faded after the booster dose.
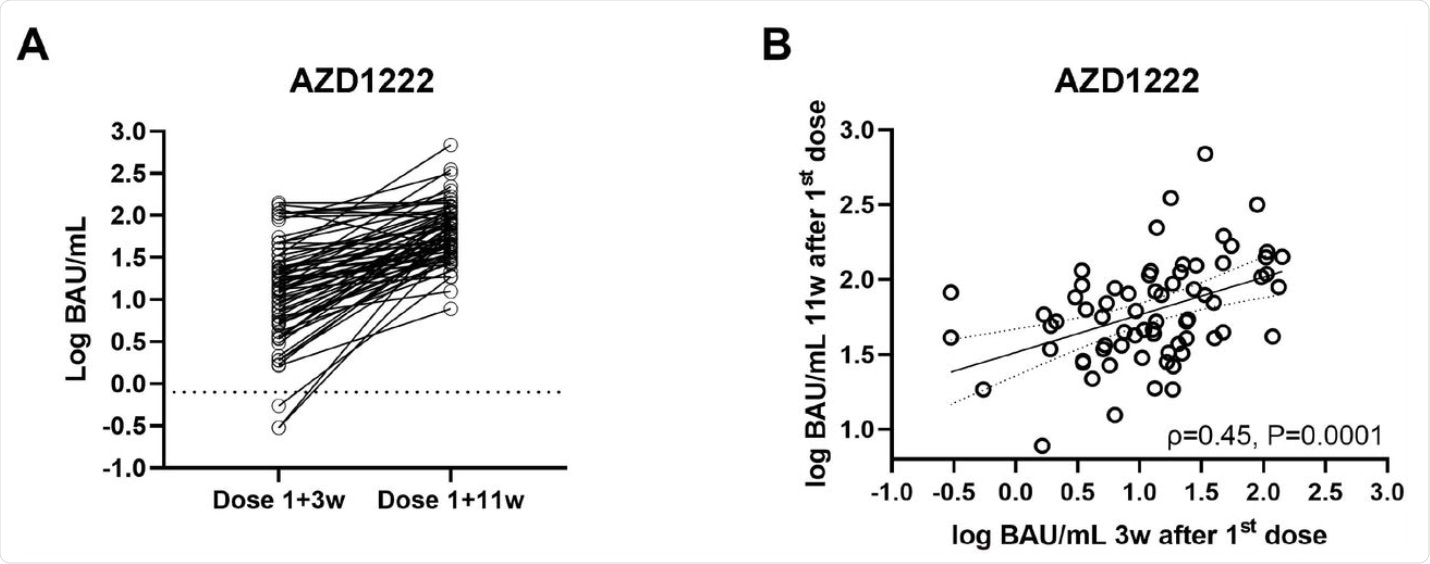 A) Intraindividual progression (A) and correlation (B) of antibody levels (Roche Elecsys S total antibody ECLIA) after AZD1222, measured 3 weeks after first vaccination and before booster (11 weeks). BAU/mL… binding antibody units per milliliter.
A) Intraindividual progression (A) and correlation (B) of antibody levels (Roche Elecsys S total antibody ECLIA) after AZD1222, measured 3 weeks after first vaccination and before booster (11 weeks). BAU/mL… binding antibody units per milliliter.
What are the implications?
With the assay used in this study, antibody levels above 15 BAU/mL were found to be strongly predictive of a neutralizing response. The scientists demonstrated a strong correlation between the initial and final antibody response after two doses of the BNT162b2 vaccine, but not with AZD1222.
This is the first time that this specific antibody response has been reported. This loss of association between the antibody titer to the first and second doses of AZD1222 could be due to the antibodies elicited by the vector virus, which is a chimpanzee adenovirus ChAdOx1 that may impair the response to the SARS-CoV-2 spike. Further research will be required to identify similar patterns with other vaccines.
The age-related increase in antibody titer values after the booster dose of AZD1222 could be due to older individuals who have already encountered the adenovirus, which promotes an unimpaired response to the S protein.
With the BNT162b2 vaccination, younger vaccine recipients showed a higher antibody response after the first dose. However, this difference was unremarkable after the second dose. This difference in antibody levels between older and younger individuals after the first dose of a COVID-19 vaccine has been found by earlier researchers as well.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Perkmann, T., Perkmann-Nagele, N., Mucher, P., et al. (2021). Initial SARS-CoV-2 vaccination response can predict booster response for BNT162b2 but not for AZD1222. medRxiv. doi:10.1101/2021.07.06.21260059. https://www.medrxiv.org/content/10.1101/2021.07.06.21260059v1.
- Peer reviewed and published scientific report.
Perkmann, Thomas, Nicole Perkmann-Nagele, Patrick Mucher, Astrid Radakovics, Manuela Repl, Thomas Koller, Galateja Jordakieva, Oswald F. Wagner, Christoph J. Binder, and Helmuth Haslacher. 2021. “Initial SARS-CoV-2 Vaccination Response Can Predict Booster Response for BNT162b2 but Not for AZD1222.” International Journal of Infectious Diseases 110 (September): 309–13. https://doi.org/10.1016/j.ijid.2021.07.063. https://www.ijidonline.com/article/S1201-9712(21)00620-2/fulltext.