Studies have shown that age and male sex are the key drivers for severe COVID-19, apart from other factors such as preexisting autoimmune conditions and malignancy. Scientists across the globe agree that the backbone of strategy that could lead to the end of this pandemic is mass vaccination. Although messenger RNA (mRNA) vaccines had high efficacy in randomized clinical trials, immunocompromised patients who might have inferior vaccination responses were not included in these trials.
B-cell depleting therapies increase morbidity and mortality in COVID-19 patients. However, evidence-based vaccination strategies are lacking for this specific population. A better understanding of humoral and cell-mediated responses to mRNA vaccines in patients treated with anti-CD20 depleting agents could help develop individualized vaccination strategies. Recent data offered evidence that cell-mediated immune responses were crucial for vaccine efficacy and may provide protection even in B cell-depleted COVID-19 patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Humoral and cell-mediated immune responses to mRNA-based vaccines in patients undergoing treatment with CD20-B-cell depleting agents
Researchers from Switzerland recently investigated humoral and cell-mediated immune responses to mRNA-based vaccines in patients undergoing treatment with CD20-B-cell depleting agents for autoimmune diseases, transplantation, or malignancy. Their study is published on the medRxiv* preprint server.
The patients chosen for this study at the Bern University Hospital had a history of treatment with anti-CD20 depleting agents such as rituximab or ocrelizumab. They were enrolled to analyze humoral and cell-mediated immune responses using the interferon-ɣ release assay after receiving the vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The primary outcome of the study was the anti-spike antibody response in 96 anti-CD20-treated patients compared to healthy controls.
“Rituximab and biosimilars are critical backbones in the treatment of patients with autoimmunity and/or B-cell mediated malignancy.”
Results show blunted humoral and cell-mediated immune responses to COVID-19 mRNA vaccines in patients with CD20-depleting treatment history
The researchers detected anti-spike IgG antibodies in 49% of patients 1.79 months after they received the second dose of the vaccine in comparison to 100% of controls. The SARS-CoV2 specific interferon-ɣ release was noted in 17% of patients and 86% of immunocompetent controls. Only 5% of patients showed positive reactions in both assays, while 86% of healthy controls had a positive reaction. Humoral vaccine response was predicted using time since last anti-CD20 therapy - 7.6 months, peripheral CD19+ of >27/µl, and CD4+ lymphocyte count - >653/µl.
In conclusion, this analysis offers evidence for blunted humoral and cell-mediated immune responses elicited by COVID-19 mRNA vaccines in patients with a history of CD20-depleting treatment. In addition, lymphocyte subpopulation counts were associated with vaccine response in this vulnerable patient population. According to the authors, their report adds novelty to the results from a series of smaller studies that previously analyzed humoral and cellular responses to SARS-CoV-2 vaccination in patients with B-cell depleting anti-CD20 therapy history.
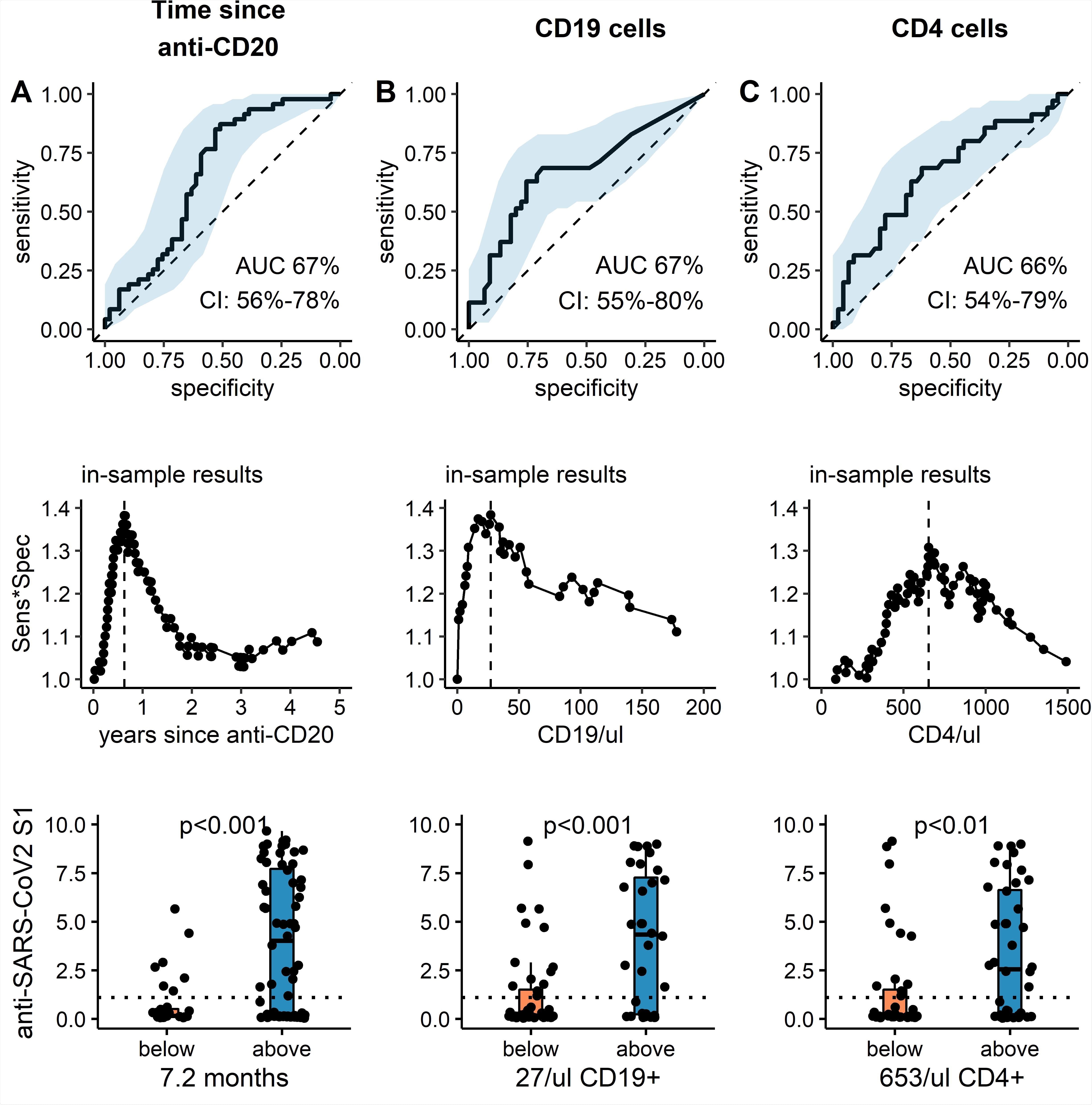
Receiver operating characteristic curve, optimized cut-off and predictive value of clinical and serological parameters to predict anti-SARS-CoV2 humoral response. (top panel): ROC curve for (A) time since last anti-CD20 treatment, (B) CD19 count and (C) CD4 count to predict dichotomous anti-SARS-CoV2 S1 IgG levels above 1.1 (Index s/c) at least 4 weeks after the second SARS-CoV2 vaccine. Black solid line: ROC curve, blue ribbon: 95% CI, dotted line: null hypothesis. (middle panel): Calculation of optimal cutoffs to predict dichotomous anti-SARS-CoV2 S1 IgG response using Youden method. Product of Sensitivity and Specificity is plotted against continuous variable: (A) time since CD20-depletion (years), (B) CD19 count and (C) CD4 count. Lower panel: Absolute anti- SARS-CoV2 S1 IgG are shown (median, IQR and min/max). For patients with parameters below (orange) or above (blue) the respective cutoff values. Additionally, individual values of each patient are plotted as single points. Dotted line: cutoff anti-SARS-CoV2 -IgG value of 1.1 (s/c). p-values: *<0.05, **<0.01.
Study identifies potential predictors for COVID-19 vaccination efficacy in anti-CD20 treated patients
In this study, although humoral responses against SARS-CoV2 mRNA vaccines were observed in all immunocompetent controls, only 49% of anti-CD20 treated patients had humoral responses to the vaccines. After stratification for the treatment indication, patients with autoimmune diseases had a higher response rate compared to patients post-transplantation or those with cancer. This might be due to differences in concomitant immunosuppressive treatment.
“Strengths of this study include the identification of potential predictors for vaccination efficacy in anti-CD20 treated patients.”
Similarly, cellular responses elicited by vaccines were seen in 17% of patients and 86% of healthy controls. Overall, while most of the healthy individuals mounted successful humoral and cellular vaccination responses, only 5% of anti-CD20 exposed patients had successful responses to vaccination. This underlines the complex sequelae of B cell depletion on B cell and T cell interactions. Apart from the expected lower CD19+ B cell counts and IgM levels, these patients also showed lower numbers of CD3+ and CD4+ T cells.
“These findings support the notion that selective B-cell depletion indirectly results in a reduction of certain subsets of T-lymphocytes, which may further impair vaccination efficacy.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Humoral and cellular responses to mRNA vaccines against SARS-CoV2 in patients with a history of CD20-B-cell depleting therapy Matthias B. Moor, Franziska Suter-Riniker, Michael P. Horn, Daniel Aeberli, Jennifer Amsler, Burkhard Möller, Linet M. Njue, Cesare Medri, Anne Angelillo-Scherrer, Luca Borradori, Susanne Radonjic-Hoesli, Andrew Chan, Robert Hoepner, Vera Ulrike Bacher, Laila-Yasmin Mani, Joseena Mariam Iype, Cédric Hirzel, Britta Maurer, Daniel Sidler medRxiv 2021.07.04.21259848; doi: https://doi.org/10.1101/2021.07.04.21259848. https://www.medrxiv.org/content/10.1101/2021.07.04.21259848v1
- Peer reviewed and published scientific report.
Moor, Matthias B, Franziska Suter-Riniker, Michael P Horn, Daniel Aeberli, Jennifer Amsler, Burkhard Möller, Linet M Njue, et al. 2021. “Humoral and Cellular Responses to MRNA Vaccines against SARS-CoV-2 in Patients with a History of CD20 B-Cell-Depleting Therapy (RituxiVac): An Investigator-Initiated, Single-Centre, Open-Label Study.” The Lancet Rheumatology 3 (11): e789–97. https://doi.org/10.1016/S2665-9913(21)00251-4. https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(21)00251-4/fulltext.