The three major COVID-19 vaccines, Moderna, Pfizer-BioNTech, and AstraZeneca, are based on two technologies: messenger RNA (mRNA) delivered by liposomes for the two former or delivery of the spike protein by a replication-deficient vector, in the case of the latter. Therefore, it is strongly recommended that an individual receive both first and second doses of the same type of vaccine, though in some cases, a mixed dosing regimen has been prescribed.
A higher prevalence of side-effects in particular groups after receiving the AstraZeneca vaccine caused those waiting to receive their second dose to be advised to switch to an mRNA-based vaccine in some locations. Animal studies show that good humoral and cellular immunity is imparted under a heterologous vaccination regimen, though often with a higher prevalence of short-term side effects.
The validity and safety of mixing first and second doses of different types are explored in a paper recently uploaded to the preprint server medRxiv* by Rose et al. (July 13th, 2021), finding that robust antibody response is induced, comparable to, and perhaps even exceed that generated by homologous dosing.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
How was the study performed?
Fifty-nine participants were recruited for the study, 42 of which received a heterologous immunization scheme, the majority of which were in the order: AstraZeneca - Pfizer-BioNTech. The remaining participants received either the AstraZeneca or Pfizer-BioNTech vaccine alone. Serum samples were collected before receiving the second dose and two weeks after, subsequently undergoing three types of commercially available SARS-CoV-2 Immunoglobulin G (IgG) enzyme-linked immunosorbent assay to assess the immune response in each individual.
The sera were also compared against two SARS-CoV-2 neutralization assays, wherein the ACE2 receptor is bound to the solid phase of the assay with the receptor-binding domain of the spike protein-free in solution. The neutralization potency of the antibodies can then be assessed by determining those that cause the most significant reduction in bonding between the molecules.
How did the vaccines perform?
Following administration of the first dose of either the mRNA or AstraZeneca vaccines, all participants saw an increase in anti-spike protein antibodies according to each of the assays employed, rising to 100% IgG prevalence after the second dose in each case. Interestingly, mean IgG titers following a heterologous vaccine regimen were 7-10 times higher than those observed in the AstraZeneca only group, while those receiving the Pfizer-BioNTech vaccine only were 10-15 times higher.
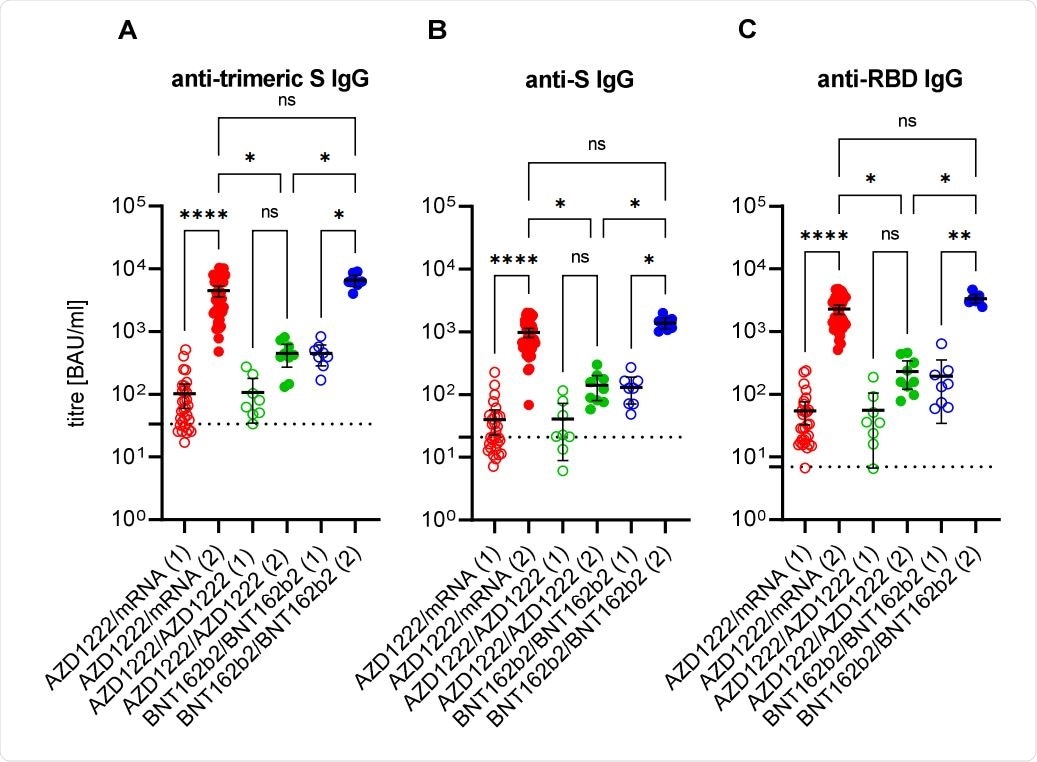
Anti-SARS-CoV-2 immunoglobulin G response after first (1; empty circles) and second (2; filled circles) immunisation with the vector vaccine AZD1222 or the messenger ribonucleic acid (mRNA)-based vaccines BNT162b2 or mRNA-1273. The cut-offs for positivity (including borderline results) of anti-trimeric spike (S) IgG assay (A), of the anti-S IgG assay (B), and of the anti-RBD IgG assay (C), respectively, are marked by dashed lines. Ns: non-significant; *: p < 0.05; **: p < 0.01; ****: p < 0.0001 (adjusted non-paired and non-parametric test; Kruskal-Wallis test).
Similarly, low to intermediate potency between serum-derived antibodies was observed following administration of the first dose of any vaccine, becoming very high after the second. A mixed-dose type regimen generated 13 higher fold mean virus-neutralizing antibodies than the AstraZeneca vaccine alone and a slightly higher titer than induced by the Pfizer-BioNTech vaccine alone.
The authors highlight that other studies comparing heterologous administration between the vector-based AstraZeneca vaccine and mRNA-based Pfizer-BioNTech vaccine show little difference in IgG response, and that yet further studies have suggested a 9-fold increase in anti spike antibodies under mixed dosing compared with the vector-based vaccine alone, as was observed here in both cases.
Only one other study in humans has yet compared these administration strategies, focusing on comparison with the mRNA vaccine and also noting similar results.
As mixed dosing between the vector-based vaccine and mRNA-based vaccine results in comparable or surpassing antibody response to the mRNA vaccine alone, as suggested by this study, heterologous immunization may provide a way in which the usually less effective vector-based vaccine can be supplemented, also potentially avoiding the additional side effects seen to be incurred by this vaccine.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.