Alzheimer’s disease impacts over 30 million people across the globe, yet the factors underlying its etiology remain unclear. Recent research has turned to the gut, exploring its potential role in Alzheimer’s progression. In a recent study published in the journal Science Advances, a team of researchers from Italy and France used advanced imaging techniques to explore the gut-brain axis and uncovered significant gut alterations in three distinct Alzheimer’s disease mouse models, shedding light on the disease’s complex nature.
Background
Alzheimer's disease is the leading cause of dementia and is characterized by cognitive decline and brain deterioration. Despite extensive research, its precise causes remain unclear, and current treatments offer limited relief. Recent studies have highlighted the gut-brain axis — the communication pathway linking gut health and brain function — as a potential contributor to Alzheimer’s disease.
The gut microbiome plays a crucial role in maintaining overall health and has been linked to cognitive function. While past research suggests that dysbiosis—an imbalance in gut bacteria—may influence Alzheimer's progression, this particular study focused on structural and cellular alterations in the gut rather than microbial composition. Evidence also suggests that disruptions in gut microbiota may promote inflammation and enable harmful bacteria to reach the brain.
Furthermore, changes in gut morphology have been observed in Alzheimer’s disease patients and animal models, suggesting a possible connection between gut health and neurodegeneration.
About the study
With the hope that understanding these interactions between the gut microbiome and the brain may open new avenues for early diagnosis and innovative treatments for Alzheimer's disease, the present study investigated gut alterations in Alzheimer's disease models using advanced imaging techniques, specifically both micro- and nano-three-dimensional (3D) X-ray phase-contrast tomography (XPCT), a breakthrough imaging method capable of high-resolution, non-invasive structural visualization.
The research team examined the gut of three distinct mouse models of Alzheimer's disease: APP/PS1 and APP23 mice, which carry human genetic mutations linked to familial Alzheimer's, and the SAMP8 model, which mimics sporadic, age-related neurodegeneration. These were compared against healthy controls. The ileum, a section of the small intestine, was chosen for its previously observed involvement in Alzheimer's pathology.
The XPCT method allowed for non-invasive, high-resolution, three-dimensional imaging, revealing intricate details of gut structures without requiring tissue staining or sectioning. Different resolutions were employed to capture detailed anatomical structures, from the cellular to the whole-organ level. Moreover, the imaging process involved capturing thousands of projections, which were then reconstructed into 3D volumes for analysis. This approach enabled visualization of gut features such as villi, crypts, and various cell types, including Paneth and goblet cells.
Furthermore, this study was among the first to use XPCT to identify changes in telocytes, a type of interstitial cell involved in tissue repair, suggesting disruptions in gut homeostasis in Alzheimer's disease. The XPCT also allowed for the identification of enteric nervous system components such as the myenteric and submucosal plexuses. Additionally, the study provided new insights into immune responses in Alzheimer's disease by examining Peyer’s patches and isolated lymphoid follicles, key gut-associated lymphoid tissues involved in immune surveillance.
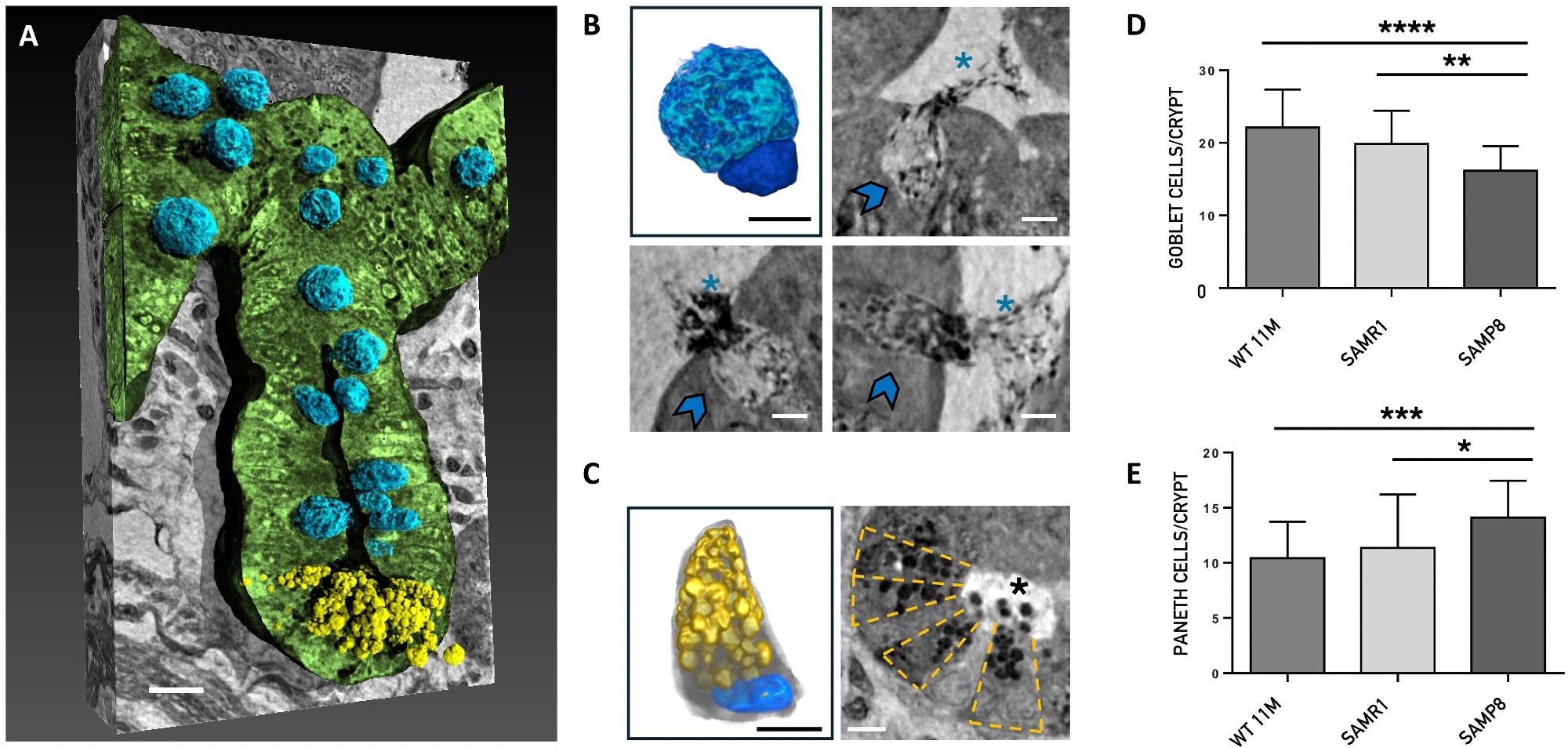
Nano-XPCT analysis of the Paneth and goblet cells. (A) Representative 3D rendering of the longitudinal view of one crypt of SAMR1 mouse. The epithelial layer of the crypt has been rendered in green. The Paneth cells are colored in yellow and the goblet cells in blue. Scale bars, 5 μm. (B) and (C) show 3D renderings and nano-XPCT close-ups of goblet and Paneth cells, respectively. The same color code of (A) has been used for the 3D renderings shown in (B) and (C). In detail, the goblet cell nucleus is colored in dark blue and the apical portion, expanded with mucin-secreting granules, which extends into the intestinal lumen, is rendered in light blue. The Paneth cell presents the typical pyramidal shape with basally situated nucleus (blue) and prominent apical granules (yellow) that occupy most of its cytoplasmatic region. Nano-XPCT close-ups in (B) show goblet cells (indicated by arrows) secreting mucus (asterisks) in the intestinal lumen. Scale bars, 2.5 μm. In (C), transversal view of a crypt in which the Paneth cells are arranged in a radial pattern (highlighted by the dashed-line boxes). The release of the antimicrobial granules (black dots, asterisk) into the lumen is visible. Scale bars, 5 μm. (D and E) Quantification of goblet and Paneth cells in the crypts. Results are obtained on 30 crypt per mouse (n = 1) and are shown as mean ± SD. One-way ANOVA P < 0.0001; post hoc by Tukey’s post hoc test: *P < 0.02, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Insights and implications
The study found that Alzheimer’s disease is associated with significant structural changes in the gut. Advanced imaging revealed alterations in the gut's villi and crypts, which are crucial structures for nutrient absorption and immune response.
In mouse models of Alzheimer’s disease, the villi appeared elongated and the crypts deeper compared to healthy controls, indicating disrupted intestinal architecture. Additionally, the intestinal epithelial barrier was significantly thinner, which, the researchers believe, could potentially compromise the gut's protective functions and increase permeability.
Furthermore, the abundance of Paneth and goblet cells, which play roles in immune defense and mucus secretion, was notably altered in Alzheimer’s models. An increase in these cells, along with heightened mucus release, was also detected, suggesting a response to inflammatory conditions in the gut. These findings align with previous observations that gut dysfunction in Alzheimer's disease may involve an inflammatory component.
The study also identified changes in the enteric nervous system, with alterations in neuron structure potentially impacting gut motility and signaling to the brain. Additionally, the morphology and numbers of telocytes were altered, suggesting impairments in gut repair mechanisms.
Moreover, the study found significant changes in Peyer’s patches and isolated lymphoid follicles, crucial for gut immune surveillance, indicating that Alzheimer’s disease may provoke a heightened immune response within the gut.
Conclusions
Overall, the research demonstrated that Alzheimer’s disease affects not only the brain but also the gut's structural integrity and immune function. This study highlighted the potential link between gut alterations and Alzheimer’s disease and the importance of gut health in neurodegenerative disorders.
Furthermore, by revealing structural changes and immune responses in the gut, the researchers suggested that these alterations could serve as early biomarkers of Alzheimer's disease. Understanding these gut-brain interactions could lead to innovative treatments aimed at mitigating Alzheimer’s progression, further emphasizing the importance of holistic approaches in addressing complex neurological diseases.
Journal reference:
- Palermo, F., Marrocco, N., Dacomo, L., Grisafi, E., Moresi, V., Sanna, A., Massimi, L., Musella, M., Maugeri, L., Bukreeva, I., Fiordaliso, F., Corbelli, A., Junemann, O., Eckermann, M., Cloetens, P., Weitkamp, T., Gigli, G., Kerlero, N., Balducci, C., & Cedola, A. (n.d.). Investigating gut alterations in Alzheimer’s disease: In-depth analysis with micro- and nano-3D X-ray phase contrast tomography. Science Advances, 11(5), eadr8511. DOI:10.1126/sciadv.adr8511, https://www.science.org/doi/10.1126/sciadv.adr8511