Antibodies elicited by natural infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or by vaccines against coronavirus disease 2019 (COVID-19) may sometimes exert a deleterious effect on the individual. The pathogenesis of these adverse effects remains unclear, and safe effective therapies are also woefully lacking.
“Compared to existing antiviral drugs, the formulation has a unique [mechanism of action] MOA of targeting receptors, broad spectrum of indications, excellent safety profile, resistance to mutations, and can be easily produced.”
 Study: A drug candidate for treating adverse reactions caused by pathogenic antibodies inducible by COVID-19 virus and vaccines. Image Credit: ktsdesign / Shutterstock.com
Study: A drug candidate for treating adverse reactions caused by pathogenic antibodies inducible by COVID-19 virus and vaccines. Image Credit: ktsdesign / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibody-dependent auto-attack
In many cases, recovery from COVID-19 is followed by weeks or months of a lingering illness, a condition that is commonly referred to as long COVID. These ‘long-haulers’ find it difficult to cope with daily life or work demands.
Vaccines have been hailed as the only way out of the COVID-19 pandemic; however, vaccines have been associated with adverse reactions and even deaths in a few rare cases. If there was a way to treat such reactions, vaccines would be much more appealing to those who are vaccine-hesitant.
The researchers of the current study previously reported a mechanism of SARS-CoV-2 infection whereby some anti-spike antibodies target host cells, which initiates an auto-immune process. This triggers serious consequences, including the deadly acute respiratory distress syndrome (ARDS) and the well-known cytokine storm that indicates a dysregulated immune-inflammatory response.
In many cases, this reaction causes the death of the patient. Dubbed “Antibody-Dependent Auto-Attack (ADAA)” by these scientists, ADAA depends entirely on the activity of the spike-specific antibodies. In fact, pregnant mice have been shown to miscarry, or have stillbirths or neonatal deaths, when exposed to anti-spike COVID-19-induced antibodies.
Mechanism of action
The cell membrane is rich in sialic acid (SA), which masks the cell surface antigens to allow for self-recognition or immune tolerance of self-antigens. The loss of SA causes autoantibodies to form, which can contribute to the activation of an immune response.
SARS-CoV-2 has been shown to possess a receptor-destroying enzyme (RDE) that strips SA from the lung epithelial cells. Replacing these SA residues would thus block the autoimmune process and prevent the binding of pathogenic antibodies to these vulnerable cells.
The researchers developed an SA analog for this very purpose. To this end, the current study reports its efficacy in preventing and treating adverse reactions caused by this mechanism.
Drug formulation
A successful SA analog is determined by its ability to enter the cell and participate in building a carbohydrate chain. Natural SA cannot do this, as it undergoes rapid breakdown in vivo. The scientists, therefore, chose to use N-acetylneuraminic acid methyl ester (NANA-Me) as their SA analog.
NANA-Me is an N-acetylneuraminic acid (NANA) analog that is often preferred over NANA for its cell entry capabilities. However, NANA-Me is unstable at a neutral pH and requires environmental conditions to be more acidic, at a pH of around 4-4.5.
The researchers therefore designed a formulation with NANA-Me:NANA in the 2:1 ratio called BH-103. When cultured with lung epithelium cells, this compound increased SA levels on the cell surface in a dose-dependent manner, unlike those treated with NANA. This ratio was also seen to be optimal for the repair of SA-poor cell surfaces.
In vitro testing
SA loss was artificially induced by exposing the cells to neuraminidase or sialidase. When these injured cells were then treated with BH-103, SA levels were found to be significantly higher as compared to controls. Thus, this in vitro experiment showed that BH-103 may be able to increase SA levels in damaged cells and ultimately prevent the autoimmune phenomena from occurring to reduce disease severity.
Viral entry of SARS-CoV-2 depends on spike-mediated viral engagement with the angiotensin-converting enzyme 2 (ACE2) host cell receptor, via the spike receptor-binding domain (RBD).
Using recombinant SARS-CoV-2 RBD, the researchers found that treatment with BH-103 improved SA levels on exposed cells as compared to controls. Simultaneously, however, the exposed cells had lower levels of spike-RBD compared to controls.
When neuraminidase- or sialidase-treated cells were cultured with this compound, SA levels fell, but less than that which was observed in untreated cells. However, the spike-RBD binding to the treated cells was reduced by almost 90%, despite the SA loss. In other words, the compound reduced virus binding.
“This result indicated that replacement of NANA by NANA-Me induced a structural change or chemical modification of the viral receptor. BH-103 not only repaired the missed sialic acid but also significantly decreased the binding affinity of the COVID-19 S-RBD simultaneously.”
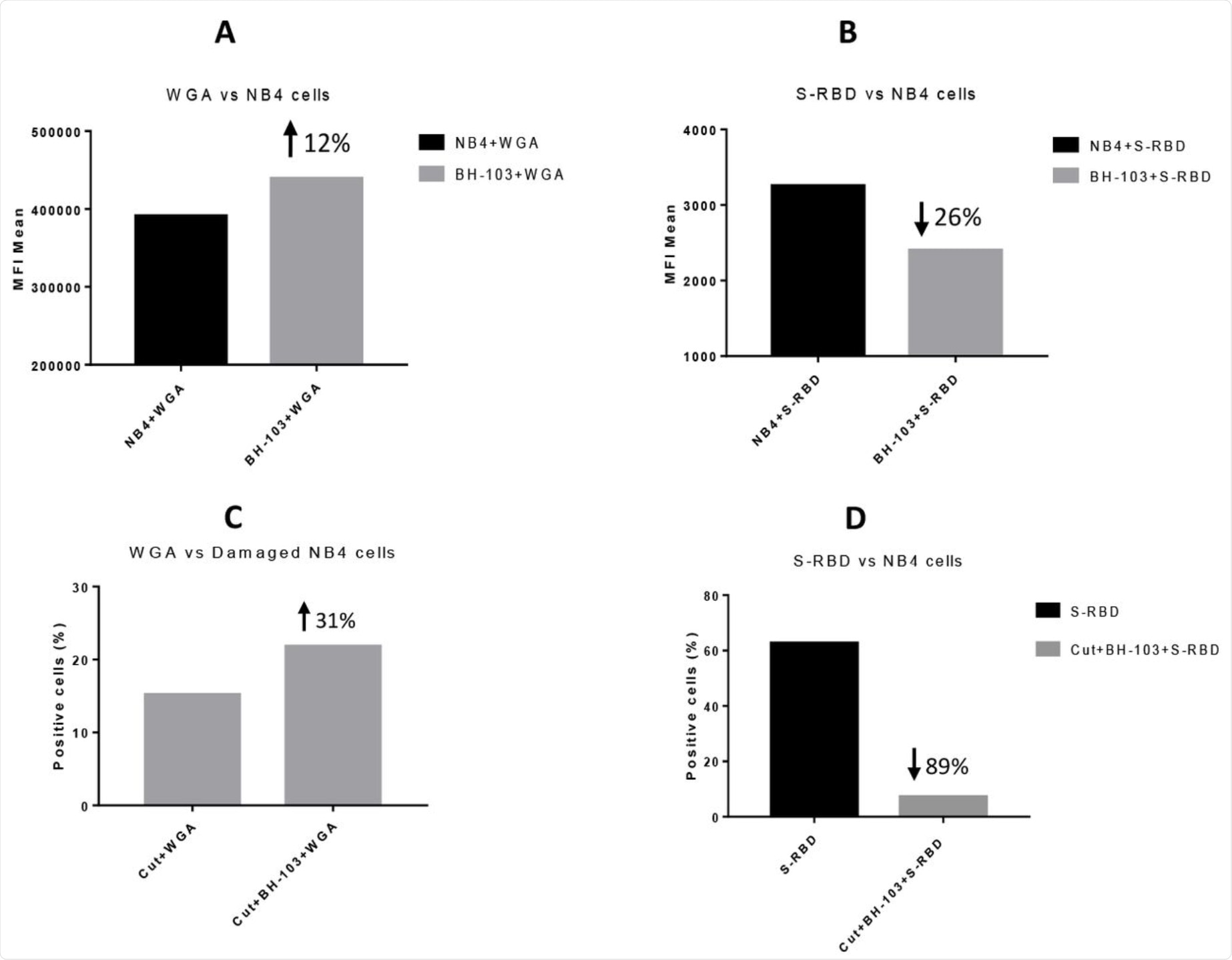 The levels of sialic acid or S-RBD of SARS-CoV-2 virus on the surface of acute promyelocytic leukemia NB4 cells, determined by a flow cytometry analysis. A: NB4 cells were treated with or without BH-103; B: Binding of S-RBD to NB4 cells with or without BH-103 treatment; C: Damaged NB4 cells (sialidase treated) were treated with or without BH-103; and D: Binding of S-RBD to damaged NB4 cells with or without BH-103 treatment.
The levels of sialic acid or S-RBD of SARS-CoV-2 virus on the surface of acute promyelocytic leukemia NB4 cells, determined by a flow cytometry analysis. A: NB4 cells were treated with or without BH-103; B: Binding of S-RBD to NB4 cells with or without BH-103 treatment; C: Damaged NB4 cells (sialidase treated) were treated with or without BH-103; and D: Binding of S-RBD to damaged NB4 cells with or without BH-103 treatment.
In vivo testing
Using a mouse model, the researchers injected spike-specific antibodies into pregnant mice twice a day every three days, on days 15 and 18, respectively. The mice were also treated with BH-103 just before or along with these antibody injections.
BH-103 was again found to significantly reduce the rate of disease and fatality induced by the pathogenic antibodies. This was confirmed by the significant histologic improvement seen in the mouse organs including the lungs, kidneys, heart, and brain, as compared to the control mouse pups. BH-103 is therefore able to prevent and treat inflammatory injury to multiple organs induced by pathogenic antibodies to SARS-CoV-2.
Cytokine levels in the treated mouse pups were also lower than in the controls, though higher than normal. This data indicates that the use of BH-103 is also able to mute the cytokine storm caused by the virus.
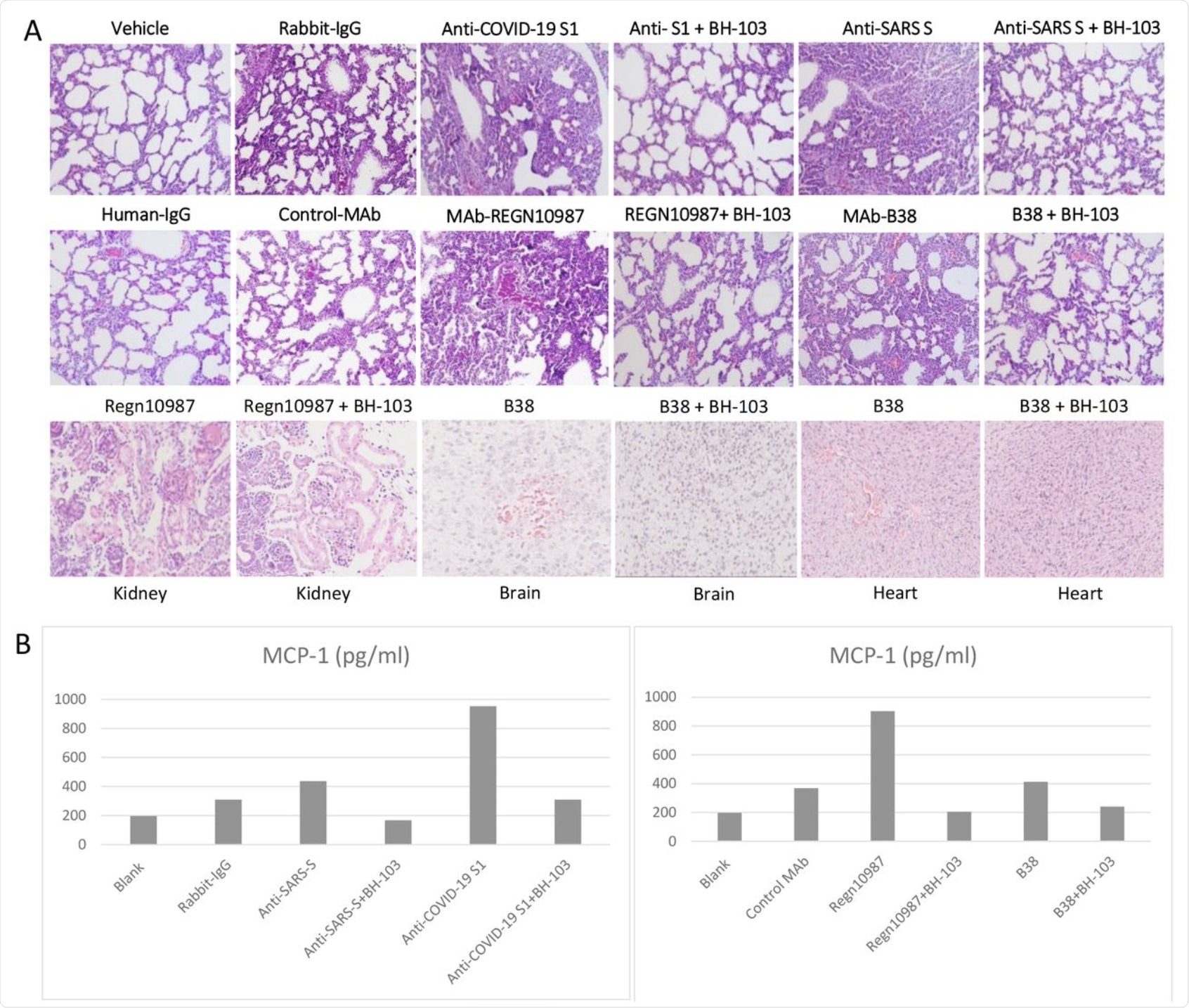 A: The representative images of the histological changes of lungs (top 2 rows), kidneys, brains, and hearts (bottom row) from the newborn mouse pups born to the dames injected with pathogenic anti-coronavirus antibodies of anti-Covid-19 S1, anti-SARS S, and human monoclonal anti-COVID-19 S1 antibodies (MAb) REGN-10987 and B38, and control antibodies of human IgG, rabbit IgG and CR3022-b6 (Control MAb); or the dames treated with BH-103 and antibody. B: The cytokine levels of MCP-1 in mouse sera from the newborn mouse pups born to the dames with antibody injection alone or the dames treated with BH-103 and antibody.
A: The representative images of the histological changes of lungs (top 2 rows), kidneys, brains, and hearts (bottom row) from the newborn mouse pups born to the dames injected with pathogenic anti-coronavirus antibodies of anti-Covid-19 S1, anti-SARS S, and human monoclonal anti-COVID-19 S1 antibodies (MAb) REGN-10987 and B38, and control antibodies of human IgG, rabbit IgG and CR3022-b6 (Control MAb); or the dames treated with BH-103 and antibody. B: The cytokine levels of MCP-1 in mouse sera from the newborn mouse pups born to the dames with antibody injection alone or the dames treated with BH-103 and antibody.
What are the implications?
Taken together, the novel drug candidate BH-103 appears to be effective in repairing SA loss through the use of a unique mechanism of action to modify the virus receptor. Through this mechanism, BH-103 is capable of reducing binding affinity, while simultaneously remaining stable under neutral environmental conditions. These findings suggest that this formulation may be developed into a useful drug to prevent and treat disease caused by COVID-19-related antibodies.
Furthermore, the MOA of BH-103 may have a broad spectrum of efficacy, as it acts on all viruses that share this receptor and is not easily blocked by escape mutations. In addition, BH-103 appears to reduce multiple symptoms traceable to ADAA, including cytokine storm in the acute phase and long-haul symptoms in the later phase of convalescence. Vaccine-related adverse effects and autoimmune manifestations related to COVID-19 are also addressed.
The safety of BH-103 appears to be established in animal models. This compound is also easily scalable on a commercial basis, as it is associated with a simple and rapid manufacturing process.
“Therefore, BH-103 will provide an effective resolution for the urgent clinic unmet of the COVID-19 pandemic and the other possible pandemic emerging in future.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Wang, H., Wu, X., Zhang, Y., et al. (2021). A drug candidate for treating adverse reactions caused by pathogenic antibodies inducible by COVID-19 virus and vaccines. bioRxiv. doi:10.1101/2021.07.13.452194. https://www.biorxiv.org/content/10.1101/2021.07.13.452194v1
- Peer reviewed and published scientific report.
Wang, Huiru, Xiancong Wu, Lin Dai, Yue Hu, Yuekai Zhang, Yuxing Chen, and Xiaoling Liu. 2022. “A Drug Candidate for Treating Adverse Reactions Caused by Pathogenic Antibodies Inducible by SARS-CoV-2 Virus and Vaccines.” Journal of Biotechnology and Biomedicine 5 (2): 63–77. https://www.fortunejournals.com/articles/a-drug-candidate-for-treating-adverse-reactions-caused-by-pathogenic-antibodies-inducible-by-sarscov2-virus-and-vaccines.html.