The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel coronavirus that was first reported in Wuhan, China in December 2019. SARS-CoV-2, which has a very high rate of transmission, is the causal agent of the coronavirus disease 2019 (COVID-19).
Several vaccines have been developed against SARS-CoV-2, several of which have received emergency use authorization (EUA) from global regulatory bodies. As a result, vaccination programs have commenced in many countries, with over 3.6 billion doses that have been administered worldwide as of July 19, 2021.
 Study: SARS-CoV-2 variants of concern exhibit reduced sensitivity to live-virus neutralization in sera from CoronaVac vaccinees and naturally infected COVID-19 patients. Image Credit: Foxeel / Shutterstock.com
Study: SARS-CoV-2 variants of concern exhibit reduced sensitivity to live-virus neutralization in sera from CoronaVac vaccinees and naturally infected COVID-19 patients. Image Credit: Foxeel / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 has mutated many times since it was originally identified at the end of 2019. This has led to the emergence of various SARS-CoV-2 variants that are often referred to as variants of concern (VOCs). Several SARS-CoV-2 VOCs are associated with greater infectivity and higher mortality rates than the original SARS-CoV-2 strain. Furthermore, some of these variants have been shown to evade vaccine-induced or naturally (COVID-19 recovered patent)-induced immunity, thus posing a threat to the efficacy of available vaccines.
Effectiveness of CoronaVac vaccine against SARS-COV-2
The CoronaVac vaccine has been developed by Sinovac Biotech in China and is based on the inactivated whole-virus. This vaccine has been approved for emergency use in Thailand and has also been used in vaccination programs in many low-income countries.
The Phase 1/2 clinical trial results for CoronaVac have recently been published. In Chile, for example, a large observational study was conducted and revealed that the CoronaVac vaccine is almost 66% effective in preventing COVID-19. This study also showed that CoronaVac reduced hospitalizations by 87.5%, intensive care unit (ICU) admissions by 90.3%, and mortality rates by 86.3%.
CoronaVac vaccine vs. natural immunity
The VOCs that are currently circulating in Thailand include the B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta) strains. A new study published on the preprint server medRxiv* evaluates the efficacy of vaccine- and infection-induced antibodies against these variants.
In this study, the researchers assessed S1-receptor-binding domain (RBD)-binding immunoglobulin G (IgG) levels, and neutralizing antibody (NAb) titers against the SARS-CoV-2 prototypic vaccine strain (WT), as well as the Alpha, Beta, and Delta SARS-CoV-2 variants. The efficacy of the CoronaVac was assessed using the sera from healthcare workers who were fully vaccinated with the CoronaVac vaccine.
The sera of vaccinated individuals were compared with the sera of unvaccinated individuals who were naturally infected with COVID-19. The authors denoted COVID-19 hospitalized patients from March 2020 to May 2020 as ‘Natural Infection 2020,’ and those from April to May 2021 as ‘Natural Infection 2021.’
Taken together, individuals who received a full 2-dose regimen of CoronaVac exhibited SARS-CoV-2 S1-RBD-binding IgG, which is referred to as ‘IgG titer’ levels, that were comparable to the levels detected in patients who were naturally infected by COVID-19. Notably, IgG titers were not found to be affected the age and/or gender of the vaccinated individual. However, the day on which the serum was collected from the candidates was found to be an important determining factor for their IgG titer values. All participants of the cohort were reported to be seropositive for IgG.
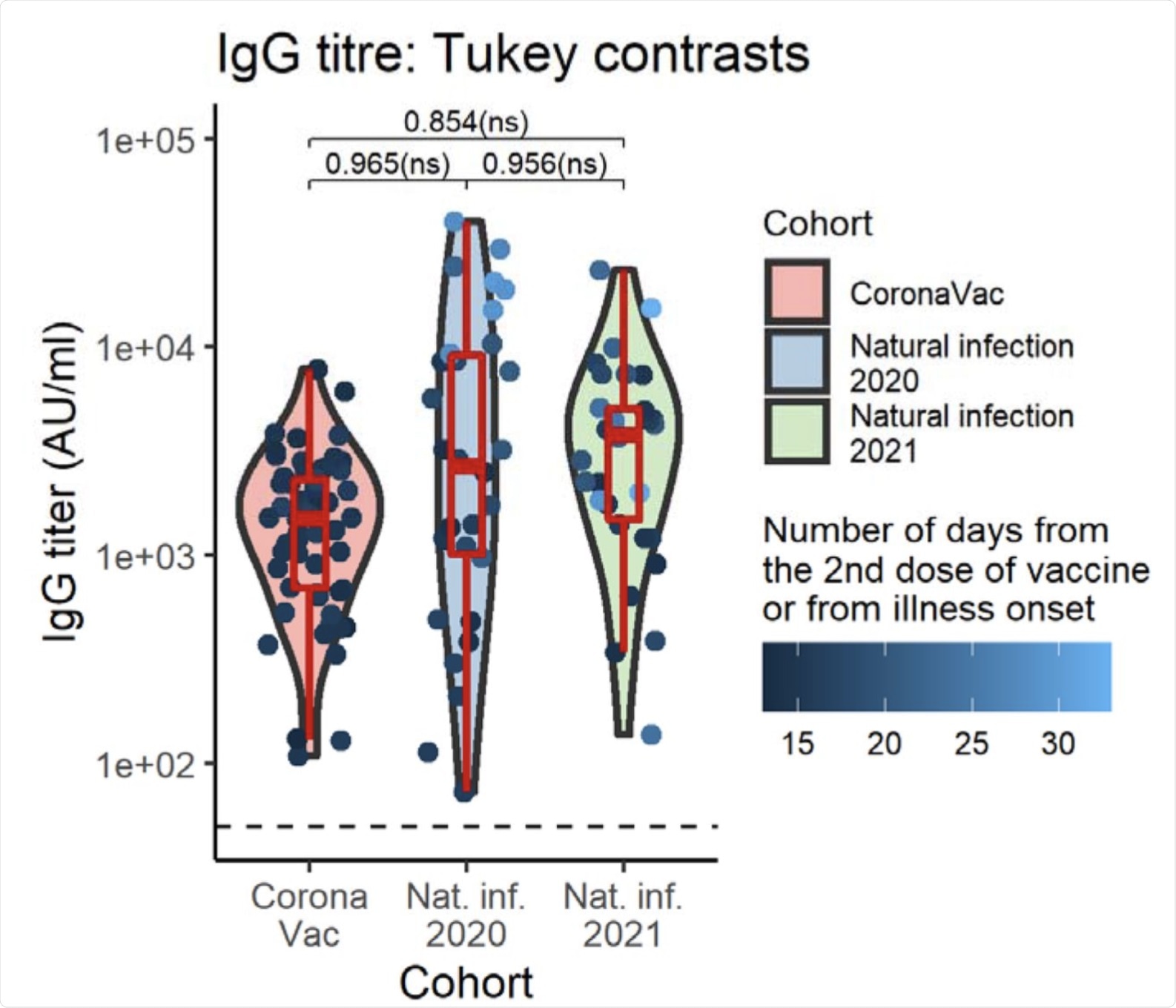 Violin plots of IgG titer by cohort group. Boxplots indicate medians and IQRs. The horizontal dotted line indicates the threshold for positive detection (50 AU/ml). There was no significant difference in SARS-CoV-2 S1-RBD-binding IgG levels between groups adjusting for patients’ sex, age, and serum collection date. Geometric mean IgG titer and 95%CI, computed at mean serum collection date (17.16 days), age (42.25 yr) averaged cross sexes: CoronaVac = 1741.28 (1240.48-2444.25) AU/ml; Natural infection 2020 = 1875.07 (1236.29-2843.89) AU/ml; Natural infection 2020 = 2034.54 (1340.47-3087.99) AU/ml. The data points are colored according to the serum collection date. The p values shown are Tukey-adjusted p-values.
Violin plots of IgG titer by cohort group. Boxplots indicate medians and IQRs. The horizontal dotted line indicates the threshold for positive detection (50 AU/ml). There was no significant difference in SARS-CoV-2 S1-RBD-binding IgG levels between groups adjusting for patients’ sex, age, and serum collection date. Geometric mean IgG titer and 95%CI, computed at mean serum collection date (17.16 days), age (42.25 yr) averaged cross sexes: CoronaVac = 1741.28 (1240.48-2444.25) AU/ml; Natural infection 2020 = 1875.07 (1236.29-2843.89) AU/ml; Natural infection 2020 = 2034.54 (1340.47-3087.99) AU/ml. The data points are colored according to the serum collection date. The p values shown are Tukey-adjusted p-values.
CoronaVac vaccine and SARS-COV-2 VOCs
The authors also evaluated NAb levels against all SARS-CoV-2 VOCs in the cohort. In their work, NAb positivity cut-off refers to the percentage of candidates with quantifiable NAb titers above or equal to 20 units.
The NAb titers were found to be highest against the wildtype (WT) SARS-CoV-2 strain (99.17%) and lowest for the Delta strain (69.17%). Comparatively, NAb titers against the SARS-CoV-2 Alpha and Beta variants were 85.83% and 82.50%, respectively. This trend was consistently found in all cohorts.
The percentage of detectable NAb titer values in individuals vaccinated with CoronaVac was found to be lowest compared to naturally infected individuals. Based on the statistical analysis of the entire cohort, a significant decrease in the mean NAb titer was observed against the four VOCs when compared to the WT.
In the case of the Alpha and Beta strains, no significant difference in the levels of the NAb titers was found. However, the NAb titer against the Delta variant was the lowest and was significantly different from the rest.
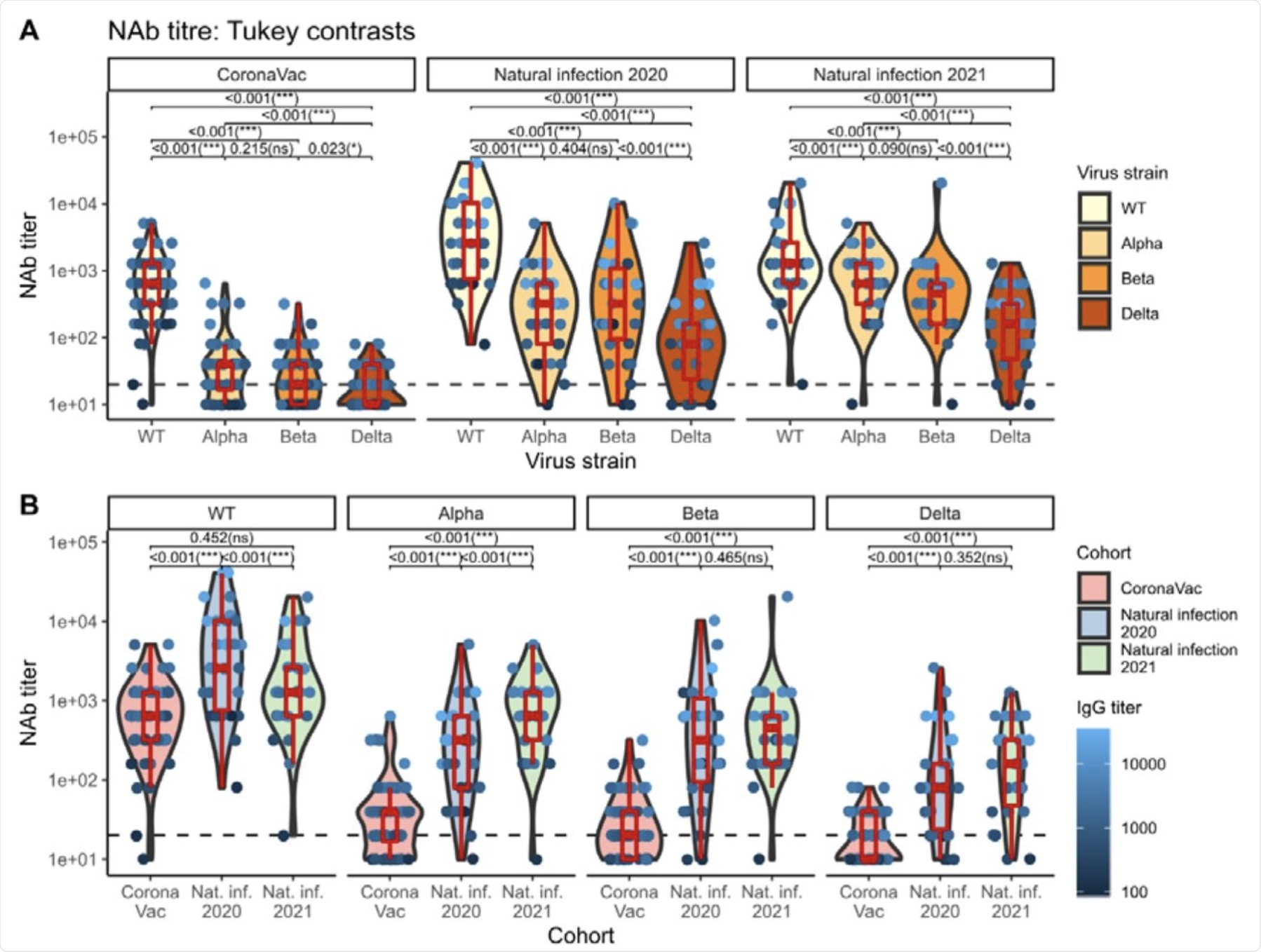 Violin plots of NAb titers grouped by virus strain nested within cohort groups (top), and by cohort group nested within virus strain (bottom). Boxplots indicate medians and IQRs. The horizontal dotted line indicates the threshold for positive detection (20 units). The data points are colored according to the IgG titer. The p-values shown are Tukey-adjusted p-values. IgG titer-, sex-, and age-adjusted geometric mean NAb titers can be found in Table 3.
Violin plots of NAb titers grouped by virus strain nested within cohort groups (top), and by cohort group nested within virus strain (bottom). Boxplots indicate medians and IQRs. The horizontal dotted line indicates the threshold for positive detection (20 units). The data points are colored according to the IgG titer. The p-values shown are Tukey-adjusted p-values. IgG titer-, sex-, and age-adjusted geometric mean NAb titers can be found in Table 3.
Conclusion
The authors of this study reported that the WT strain of SARS-CoV-2 was best neutralized by Natural Infection 2020 when compared to CoronaVac and Natural Infection 2021 sera. However, the Alpha variant was best neutralized by Natural Infection 2021 sera, when compared to CoronaVac and Natural Infection 2020 sera.
In the case of the Beta variant, this form of SARS-CoV-2 was best neutralized by Natural Infection 2020 and 2021 sera with higher NAb titer levels. Although the Delta variant was neutralized well by Natural Infection 2020 and 2021 sera, levels of NAb titers were much lower when compared with the Alpha and Beta variants.
In summary, the results of the current study indicate that NAb titers elicited by CoronaVac are much lower when compared to natural infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Vacharathit, V., Aiewsakun, P., Manopwisedjaroen, S., et al. (2021). SARS-CoV-2 variants of concern exhibit reduced sensitivity to live-virus neutralization in sera from CoronaVac vaccinees and naturally infected COVID-19 patients. medRxiv. doi:10.1101/2021.07.10.21260232. https://www.medrxiv.org/content/10.1101/2021.07.10.21260232v2
- Peer reviewed and published scientific report.
Vacharathit, Vimvara, Pakorn Aiewsakun, Suwimon Manopwisedjaroen, Chanya Srisaowakarn, Thanida Laopanupong, Natali Ludowyke, Angsana Phuphuakrat, et al. 2021. “CoronaVac Induces Lower Neutralising Activity against Variants of Concern than Natural Infection.” The Lancet Infectious Diseases 21 (10): 1352–54. https://doi.org/10.1016/s1473-3099(21)00568-5, https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00568-5/fulltext