Previous research using animal models has indicated the importance of T‐cells, particularly CD4+ and CD8+ T-cells, in protecting individuals against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19). Despite this data, the role of T-cells in the SARS-CoV-2 immune response is not fully understood.
Since December 2020, several vaccines have received approval from regulatory agencies in many countries around the world, which has allowed vaccination campaigns to begin immunizing the global population against SARS-CoV-2. While the distribution of these vaccines has significantly reduced hospitalizations and mortalities, researchers are still working to determine the duration of this vaccine-induced immune response.
To this end, several SARS‐CoV‐2 antibody detection assays have been developed and are now widely used. However, the application of T-cell-based assays is a less explored area. A user-friendly, accurate, and standardized T-cell assay would be a useful method to assess T-cell mediated immune responses to both the COVID-19 vaccines and the infection itself.
 Study: Validation and performance evaluation of a novel interferon-γ release assay for the detection of SARS-CoV-2 specific T-cell response. Image Credit: anyaivanova / Shutterstock.com
Study: Validation and performance evaluation of a novel interferon-γ release assay for the detection of SARS-CoV-2 specific T-cell response. Image Credit: anyaivanova / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Importance of T-cell response in COVID-19 patients
In some longitudinal studies of COVID-19 patients, researchers have correlated the presence of T-cells with milder COVID-19 symptoms. These studies have highlighted the importance of the T-cell response for preventing or controlling COVID-19 infection.
T-cell responses in both COVID-19 recovered patients and vaccinated individuals have also been reported to remain effective, even against SARS-CoV-2 variants of concern (VoC). As the neutralizing capacity of antibodies wanes off with time, it is extremely important to evaluate T-cell mediated reactivity to determine the effectiveness of current vaccines.
T-cell based assay for the detection of SARS-CoV-2
A new study published on the preprint server medRxiv* focuses on the evaluation of a commercially available interferon γ (IFN‐γ) release assay (IGRA) to determine T-cell responses targeted against SARS-CoV-2. Typically, IGRA is used for the detection of tuberculosis and several other pathogens.
One of the advantages associated with T-cell-based assays is that they can be performed quickly and without requiring sophisticated equipment. Another advantage is that the results of this assay are represented in international units, thus allowing for the quantification of IFN‐γ.
In the current study, SARS-CoV-2 antibody levels were evaluated in immunocompetent healthcare workers (HCW) from the Freiburg University Hospital. Whereas the first group consisted of HCW who experienced mild SARS-CoV-2 infection in 2020, the second group consisted of individuals who had been vaccinated against COVID-19. HCWs who were never infected with COVID-19 before receiving the vaccine were used as a negative control group.
To assess the assay in a real-life setting, the researchers analyzed COVID-19 vaccine-induced T-cell immune responses in different patient groups including immunocompromised patients. For this study, researchers collected blood samples of the participants to evaluate the accuracy of the novel IGRA.
Detection of IFN‐γ in COVID-19 patients
IFN-γ is the main cytokine associated with the T-cell-mediated immune response to a specific antigen, such as the spike (S) protein of SARS-CoV-2. Hence, the production of IFN-γ can be represented as a biomarker of pathogen-specific immunity.
In this study, the EUROIMMUN SARS-CoV-2 IGRA only used an S1 peptide pool for stimulation. This assay had a cut-off-dependent specificity of 96.3-100% and a reported sensitivity of 75.4-89%. The researchers revealed that this tool showed high specificity and sensitivity in the detection of T-cell immune responses in individuals after being infected with COVID-19 or post COVID-19 vaccination.
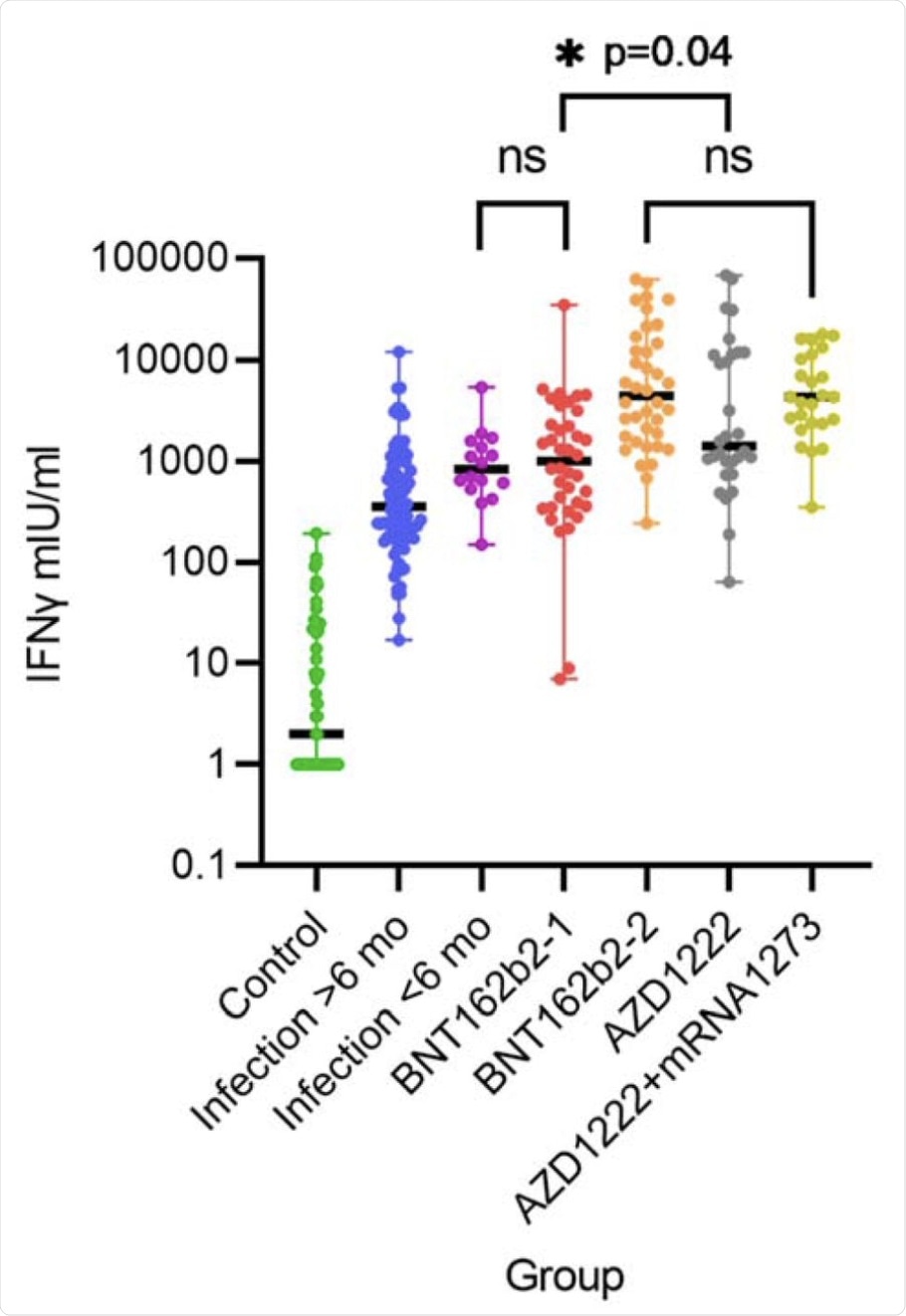 IFN-y release after stimulation with SARS-CoV-2 spike antigen in different groups. Differences are highly significant with p<0.001 unless indicated. BNT162b2-1 and -2 denotes health care workers (HCW) after the first and second dose of BNT162b2 (n=38), AZD1222 denotes HCW after first dose of AZD1222 (n=30) and AZD1222+mRNA1273 after the second dose with mRNA-1273 (heterologous vaccination scheme, n=27). ns= not significant.
IFN-y release after stimulation with SARS-CoV-2 spike antigen in different groups. Differences are highly significant with p<0.001 unless indicated. BNT162b2-1 and -2 denotes health care workers (HCW) after the first and second dose of BNT162b2 (n=38), AZD1222 denotes HCW after first dose of AZD1222 (n=30) and AZD1222+mRNA1273 after the second dose with mRNA-1273 (heterologous vaccination scheme, n=27). ns= not significant.
Using the cut-off values, this study revealed accurate readings, as indicated by the negative IGRA results found in the healthy control group. Additionally, the IGRA showed no interference with existing or cross-reactive T-cells from previous coronavirus infections.
Notably, a significant difference in T-cell responses after the first and second vaccinations, as well as between vaccines, have been observed. Another important finding of this study is that most B-cell-depleted patients showed a strong T-cell response to COVID-19 vaccination. It can therefore be concluded that these patients are likely protected from severe SARS-CoV-2 infection.
Limitation of T cell assay in diagnostics
One of the limitations associated with the use of the IGRA is the functionality of the T-cell response. To this end, the researchers found that patients who received steroid treatment for a short duration showed a negative response to SARS-CoV-2 antigens, even three weeks after receiving the messenger ribonucleic acid (mRNA) vaccine.
However, SARS-CoV-2 S1 IgG could still be detected. Therefore, it is recommended that patients who are currently receiving steroids pause their treatment prior to providing a blood sample for this type of assay.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Huzly, D., Panning, M., Smely, F., et al. (2021). Validation and performance evaluation of a novel interferon-γ release assay for the detection of SARS-CoV-2 specific T-cell response. medRxiv. doi:10.1101/2021.07.17.21260316. https://www.medrxiv.org/content/10.1101/2021.07.17.21260316v1
- Peer reviewed and published scientific report.
Huzly, Daniela, Marcus Panning, Franziska Smely, Martin Enders, Johanna Komp, Valeria Falcone, and Daniel Steinmann. 2022. “Accuracy and Real Life Performance of a Novel Interferon-γ Release Assay for the Detection of SARS-CoV2 Specific T Cell Response.” Journal of Clinical Virology 148 (March): 105098. https://doi.org/10.1016/j.jcv.2022.105098. https://www.sciencedirect.com/science/article/pii/S1386653222000348?via%3Dihub.