The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over four million deaths and infected over 194 million worldwide. In an effort to counter the rapid spread of the virus, which has an unpredictable propensity to cause severe or even fatal disease in a significant minority of cases, vaccines were developed at an unprecedentedly rapid rate.
The emergence of several SARS-CoV-2 variants that are associated with higher transmissibility and sometimes greater virulence than the wildtype SARS-CoV-2 strain has challenged the assumption that successful global vaccination can be the sole solution for combatting this virus. In fact, many of these variants have been declared variants of concern (VOCs), as they resist neutralization by antibodies directed against the virus following either natural infection or vaccination.
A new study reports that many of the VOCs and other variants that are currently in circulation, such as the B.1.526 (Iota), B.1.1.7+E484K (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and C.37 (Lambda) strains, are associated with a slightly impaired reduction in their susceptibility to neutralization by multiple antisera samples from COVID-19 vaccine recipients.
While some of these variants, such as the B.1.526 and B.1.427/B.1.429 strains are now being reported less frequently, other VOCs like the Delta variant are rapidly spreading and showing a higher prevalence in many parts of the world.
How was the study done?
In the current study, the researchers evaluated a panel of 30 sera from recipients of COVID-19 messenger ribonucleic acid (mRNA) vaccines for their neutralization of the three B.1.526 sublineages, B.1.1.7+E484K, B.1.617.2, B.1.351, and a C.37 sub-variant. The viral samples came from the wildtype isolates and from patients with COVID-19, including all the variants mentioned above.
The experimental setup allowed antibodies in the sera to interact with the viral antigens at all times, over multiple replication cycles, to simulate the conditions of actual infection. The experiment also examined binding assays against recombinant receptor-binding domain (RBD) and full-length spike proteins of several viral variants.
Reduction in neutralizing activity
The microneutralization assays showed that the sera lost neutralizing activity almost five-fold against the C.37 variant of interest. A loss in neutralizing activity was found to be approximately four-fold against the B.1.351 (beta) and B.1.1.7+E484K isolates. The latter of which lost 0.4-fold less activity than the former.
When evaluated against the Delta variant, neutralizing activity was reduced three-fold. Although conversely, a 1.4-fold reduction of neutralizing activity was reported against the Iota variant compared to the wildtype variant, it was not a significant impairment. For the E484K variant and S477N variants, neutralization activity was reduced by 1.8-fold and 2.3-fold, respectively.
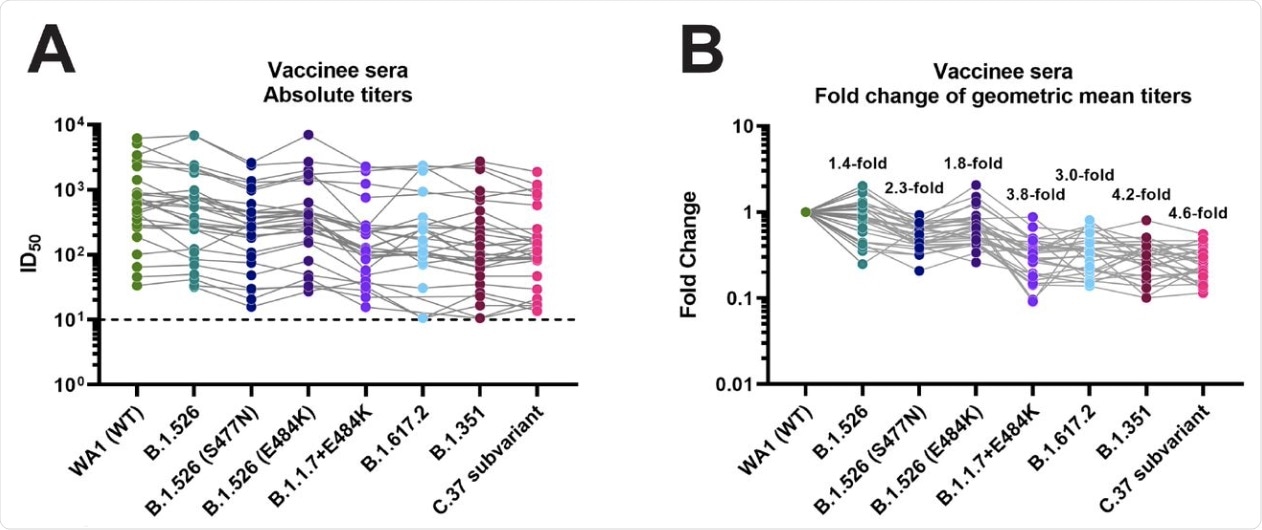 Neutralizing activity of post-mRNA vaccination sera against different VoIs and VoCs. A Absolute neutralization titers against the respective variant viruses. B Fold reduction of neutralization as compared to the wild type WA1 isolate. A list of specific mutations of the clinical isolates can be found in Supplementary Table 1.
Neutralizing activity of post-mRNA vaccination sera against different VoIs and VoCs. A Absolute neutralization titers against the respective variant viruses. B Fold reduction of neutralization as compared to the wild type WA1 isolate. A list of specific mutations of the clinical isolates can be found in Supplementary Table 1.
Antibody-binding activity
The antibody-binding activity was found to be reduced by up to 1.6-fold against the RBD of the spike protein of the Beta variant.
When tested against the full-length spike protein, no significant change was observed against the spike proteins of the B.1.1.7, B.1.351. P.1, B.1.617.1, and A.23.1 variants. The greatest reduction of 1.4-fold was reported for the Beta variant.
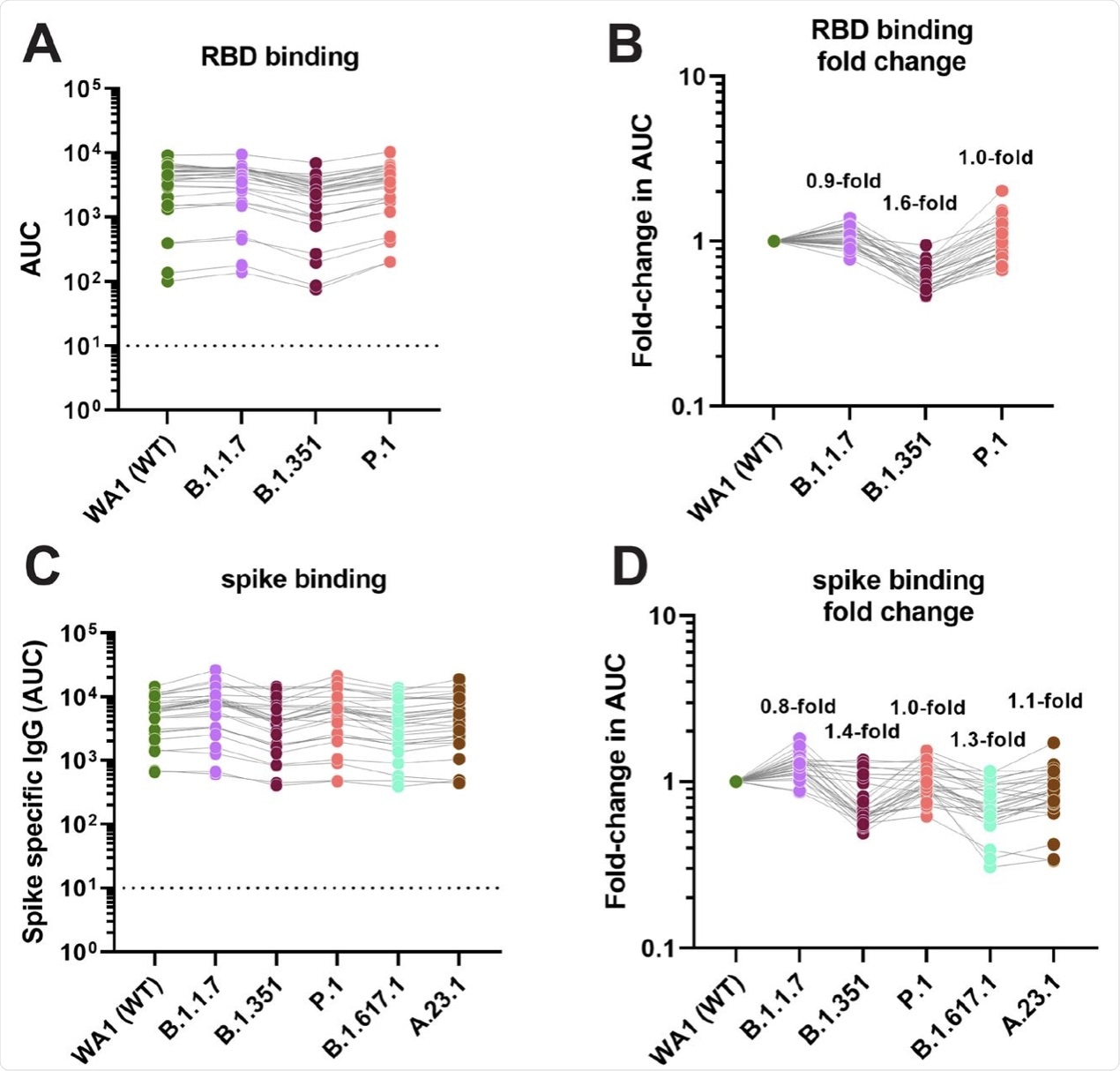 Binding activity of post-mRNA vaccination sera against RBD and spike proteins of different VoIs and VoCs. A and C show absolute area under the curve (AUC) binding values against various RBD and spike proteins, B and D show fold-reduction in binding as compare to Wuhan-1 derived wild type proteins.
Binding activity of post-mRNA vaccination sera against RBD and spike proteins of different VoIs and VoCs. A and C show absolute area under the curve (AUC) binding values against various RBD and spike proteins, B and D show fold-reduction in binding as compare to Wuhan-1 derived wild type proteins.
What are the implications?
Since neutralizing antibodies play a major role in preventing COVID-19, as shown in both animals and humans, it is important to continue surveillance of emerging variants to determine how they affect antibody-mediated neutralization.
The current study demonstrates a continuum of neutralization being effected by vaccination antisera against the several VOCs mentioned here. The smallest impact was reported on the neutralization of the B.1.526 variant, which has undergone few spike mutations relative to the wild type. The presence of E484K or S477N substitutions in the RBD of the B.1.526 variant increased the resistance towards antibody-mediated inhibition, with the latter making a greater impact.
S477N has been thought to be an escape mutation. This may account for this phenomenon occurring despite the fact that the E484K mutation in the wild-type virus, as observed with the B.1.1.7 variant, increases resistance to neutralization. Nonetheless, the Alpha variant with this mutation is associated with limited spread, perhaps because of decreased binding affinity to the human host cell receptor.
The greatest reduction in neutralizing capacity was seen not with the beta variant, as might be expected because of its three RBD mutations and several N-terminal domain (NTD) changes, but with the C.37 strain used in the study. This is attributed to the presence of additional substitutions in the N-Terminal Domain (NTD) with this strain compared to the dominant C.37 strain.
An additional finding was that the binding activity to both the spike protein and the RBD was almost intact. This is likely because there are numerous non-neutralizing epitopes on the spike.
“Interestingly, a recent study found even better correlation between binding activity and vaccine effectiveness than for neutralizing activity and vaccine effectiveness. This may indicate that binding antibodies – even if they lack neutralizing activity – are involved in protection.”
This finding corroborates reports from earlier studies on Ebola and influenza viruses discussing the protective effect of non-neutralizing antibodies acting through Fc-mediated effector functions. This points to a need for future research to confirm whether this effect is important with COVID-19 as well.
“These findings suggest that mRNA SARS-CoV-2 vaccines may remain effective against these viral variants of concern/interest and that spike binding antibody tests likely retain specificity in the face of evolving SARS-CoV-2 diversity.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Carreno, J. M., Alshammary, H., Singh, G., et al. (2021). Reduced neutralizing activity of post-SARS-CoV-2 vaccination serum against variants B.1.617.2, B.1.351, B.1.1.7+E484K and a sub-variant of C.37. medRxiv. doi:10.1101/2021.07.21.21260961.. https://www.medrxiv.org/content/10.1101/2021.07.21.21260961v1.
- Peer reviewed and published scientific report.
Carreno, Juan Manuel, Hala Alshammary, Gagandeep Singh, Ariel Raskin, Fatima Amanat, Angela Amoako, Ana Silvia Gonzalez-Reiche, et al. 2021. “Evidence for Retained Spike-Binding and Neutralizing Activity against Emerging SARS-CoV-2 Variants in Serum of COVID-19 MRNA Vaccine Recipients.” EBioMedicine 73 (November): 103626. https://doi.org/10.1016/j.ebiom.2021.103626. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00419-9/fulltext.