In the midst of a COVID-19 pandemic well into its second year, most developed countries are implementing effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine campaigns to reduce the spread of the infection.
In December 2020, the Food and Drug Administration granted the emergency use of two messenger RNA (mRNA) vaccines developed by Pfizer-BioNTech and Moderna to protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent responsible for the ongoing COVID-19 pandemic.
Phase 3 clinical trials of these vaccines previously demonstrated 94 to 95% efficacies in preventing COVID-19 and an almost 100% efficacy in protecting against severe disease.
In addition to clinical trials conducted to determine the safety and efficacy of the mRNA vaccines, additional studies began describing the serological response to the vaccines under "real-world" conditions, especially with the onset of the SARS-CoV-2 variants and case reports of vaccine escape.
"Although the initial focus may be on overall antibody levels and differences in the antibody response in previously seropositive versus seronegative vaccine recipients, other humoral antibody response factors need to be considered", says the team at the Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York.
The presence of neutralizing antibodies in people who have been previously exposed to SARS-CoV-2 versus those who have not been and binding avidity are two factors that help determine the antibody response's quality.
This study involves the evaluation of total antibody levels, antibody avidity, and neutralizing antibody levels in 49 non-infected vaccinated (NaïveVax) and 19 previously infected and vaccinated (RecoVax) healthcare workers. This response was again compared to normal post-infection antibody response in 160 non-vaccinated patients having mild symptoms (OutPtNoVax) and 122 non-vaccinated acutely infected patients (HospoNoVax) during the beginning of the pandemic.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.
What did the study involve?
The study involved retrospective and prospective study of individuals. In the retrospective study, serum was collected from patients with mild symptoms and patients with acute infections. These serums were then frozen for future analysis.
A prospective study was carried out with vaccinated individuals. In this study, blood samples were collected from participants within the first week of receiving the first dose of the Pfizer mRNA vaccine and after that two and four weeks from each vaccine dose. Participants were also asked to donate blood samples three months and six months after the first dose of the vaccine.
Next, the SARS-CoV-2 total receptor-binding domain (RBD) antibody (Tab), avidity assay, and neutralizing antibody assay were carried out. The Roche Elecsys Anti-SARS-CoV-2S and the N-antigen assay were also performed to determine the levels of nucleocapsid antigen and antibodies against the spike protein in the serum samples.
What did the study find?
The results showed that the previously infected vaccinated individuals exhibited a rapid antibody response within days after receiving the first dose. The antibody level persisted up to six months post-vaccination. The antibody response did not increase further after the second dose for this population.
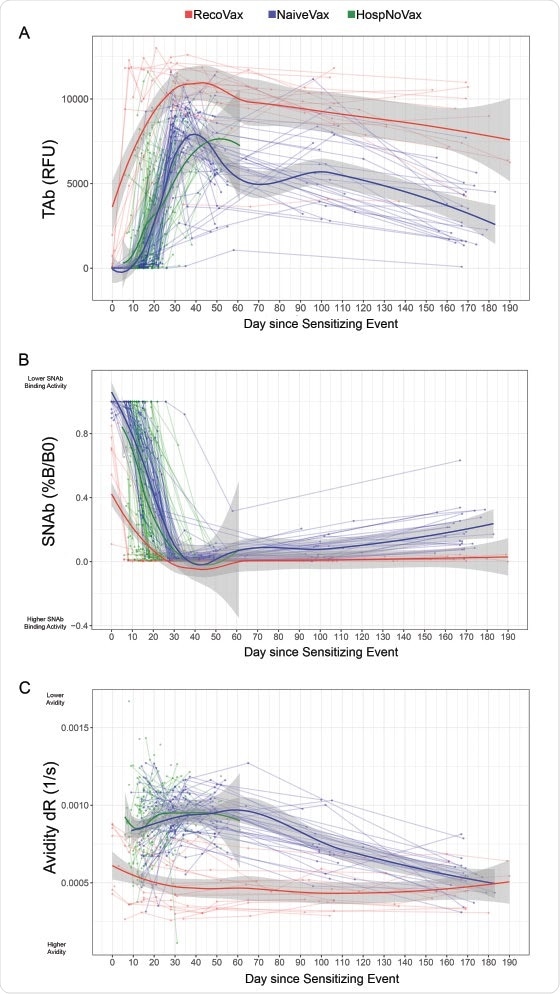
Dynamics of the anti-SARS-CoV-2 antibody response after vaccination or infection utilizing regression models. TAb (A), SNAb (B) levels and avidity (C) are displayed over time. A total of 686 data points were plotted from 19 RecoVax individuals (red), 49 NaïveVax individuals (blue) and 122 HospNoVax patients (green). All participants received the second dose 21 days after the 1st dose. The trend of antibody level overtime was described by applying Muggeo’s method of estimating regression models with unknown break-points to estimate the changing time points of the trends.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
"This is in contrast to the other cohorts, where TAb levels never fully matched RecoVax and in some cases decreased", says the team.
The neutralizing antibody levels for the RecoVax group were higher than all the other groups. They reached a maximum level after the first dose of vaccine and remained at that level for up to 6 months post-vaccination.
The RecoVax group's avidity was the highest compared to the other groups, and this level was maintained throughout the six-month follow-up period.
"Nonetheless, NaïveVax's continued avidity maturation achieved a similar avidity level by ~6 months post-D1", says the team.
What did the authors conclude?
The researchers concluded that two doses of the mRNA vaccine in NaïveVax individuals provide the same response as one dose of vaccine in RecoVax individuals.
"Individuals with mild COVID19 symptoms (OutPtNoVax) overall maintained lower antibody levels compared to the vaccinated cohorts, especially warranting vaccination despite prior infection", says the team.
It can also be concluded that since a single dose of vaccine-elicited maximum antibody response in RecoVax individuals, this dose can be considered sufficient for them.
"Monitoring individuals for antibody titers long term (as is done with the Hepatitis B or MMR vaccines), as well as monitoring neutralizing activity and avidity, may be prudent in determining the vaccine efficacy and the need for future booster vaccinations," added the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Racine-Brzostek S. E. et. Al. (2021). More rapid, robust and sustainable antibody responses to mRNA COVID-19 vaccine in convalescent COVID-19 individuals. medRxiv pre-print. doi: https://doi.org/10.1101/2021.08.04.21261561 . https://www.medrxiv.org/content/10.1101/2021.08.04.21261561v1
- Peer reviewed and published scientific report.
Racine-Brzostek, Sabrina E., Jim K. Yee, Ashley Sukhu, Yuqing Qiu, Sophie Rand, Paul D. Barone, Ying Hao, et al. 2021. “Rapid, Robust, and Sustainable Antibody Responses to MRNA COVID-19 Vaccine in Convalescent COVID-19 Individuals.” JCI Insight 6 (20). https://doi.org/10.1172/jci.insight.151477. https://insight.jci.org/articles/view/151477.