According to researchers in Texas, a lack of appropriate testing in the US has led to an underestimation of seroprevalence in the U.S., thus making it difficult to estimate population immunity to SARS-CoV-2 or vaccination.
While model-based estimation has been proposed, the calculations are based on inputs such as viral reproduction number, immune response longevity, and other dynamic factors.
In their new research paper posted to the medRxiv* preprint server, a data-driven statistical approach is presented by the scientists from The University of Texas System, which uses prospectively collected serological data along with state-level vaccination data to estimate total immunity rates using a model-based approach rather than a simplistic summing of natural- and vaccine-induced immunity.
The researchers describe a detailed procedure for reproducing their research so that policymakers can make informed decisions concerning SARS-CoV-2 locally.
The study evaluated serological data from more than 14,000 blood samples collected from 10,482 participants in a longitudinal statewide cohort survey beginning September 30, 2020.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
It is essential to estimate the number of individuals likely protected from the SARS-CoV-2 infection owing to immune responses elicited either by vaccines or natural SARS-CoV-2 infection.
Scientists can estimate total immunity using mathematical models and simulations, which require inputs like viral reproduction rate, duration of immunity once infected, population mixing, and other additional factors.
However, some of the above-stated ingredients are still unknown to the researchers. For example, some of the unknown factors include the duration of natural and vaccine-induced immunity and the influence of T-cell cross-reactivity.
In addition, the continual emergence of SARS-CoV-2 variants has threatened the effectiveness of the natural and vaccine-induced immunity against the original SARS-CoV-2 strain first reported in Wuhan, China.
Some of the recent studies, which assessed the duration of the immune response against COVID-19, revealed the presence of neutralizing antibodies for at least five months and sometimes even longer.
Additionally, these studies had also indicated a reduction in the risk of reinfection for several months in the vaccinated group or among individuals who recovered from SARS-CoV-2 infection.
Previous research conducted in Israel, where the vaccination rate has been extremely high, reported a significant drop in the SARS-CoV-2 transmission rate. Thereby, a high rate of vaccination has not only been associated with direct immune protection to the vaccinated individual but indirect protection to the unvaccinated population via the reduction in the transmission rate of the virus.
The study
This new research has demonstrated the estimation process of COVID-19 total immunity, i.e., vaccine and natural immune response against SARS-CoV-2, in Texas, USA.
The current study is based on some assumptions adopted from previous research. These assumptions are as follows: (a) reinfection within a few months of recovery from the COVID-19 infection is rare, (b) neutralizing antibodies produced after natural infections last for at least five months, and (c) vaccination provides a reasonably long-term antibody response.
In this study, the researchers used a prospectively designed serological survey to estimate the total immunity as of July 4, 2021. To this effect, researchers assessed seroprevalence, over 1-week intervals, from 14,899 blood samples obtained from participants throughout Texas. Researchers also conducted a census age-adjusted seroprevalence estimation of natural infection and pooled it with the Texas Department of State Health Services (DSHS) de-identified population-level vaccination data. This helped the authors to obtain an accurate state-level estimation of total immunity.
The mean age of the subjects was 45.9 years. This study cohort contained 60% female candidates, 88.9% white, and 92% were from urban locations. Most of the candidates were educated and were employed full-time.
The researchers estimated that the percentage of those with naturally occurring antibodies to SARS-CoV-2 in Texas is 35.3%, and the total estimated immunity is 69.1%. Therefore, this study suggests that the percentage of individuals with antibodies to SARS-CoV-2 is four times higher than the state estimate of 8.8%.
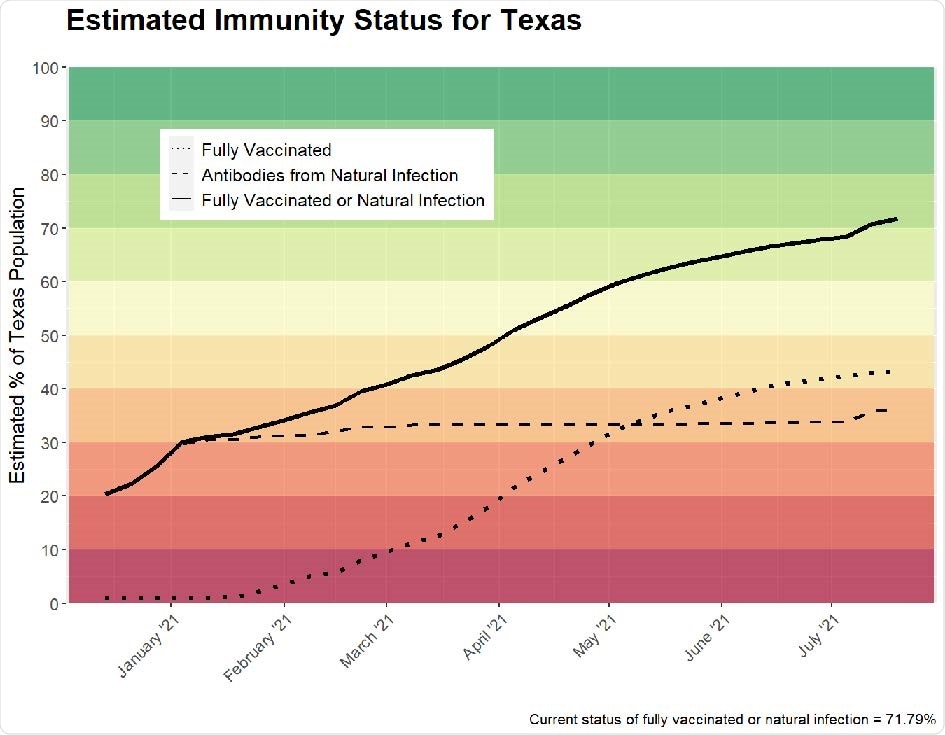
Estimated total immunity in Texas (i.e., weekly percentage of fully vaccinated or naturally occurring antibodies). Horizontal axis labels denote the first day of the month. The estimate as of July 4, 2021 is 69.08%.
According to the authors, this is the most accurate and non-model-based estimation of total immunity to date in the state of Texas.
This study did not consider confidence intervals for total immunity because the number of individuals vaccinated is a known quantity rather than an estimate. However, the confidence intervals were estimated in the case of seroprevalence, which is not known or fixed. The extensive study sample resulted in a very small range for the 95% confidence interval.
Strengths and limitations of the study
One of the limitations of this study is associated with the observational serological surveys. For instance, the sample demographic may not be an accurate representation of the state. Additionally, sampling variability or selection biases may have occurred within the small study duration of the serological survey. However, this limitation can be corrected using an appropriate time window on which several factors, such as the magnitude of the wave of infection, etc., depend.
This study considered an isotonic restriction and assumed that seroprevalence does not decrease rapidly within a short duration. This assumption nullified the issues regarding daily or weekly sampling variability.
A strong point of this study is that the researchers have considered the age variable while estimating serological and vaccination rates. This is extremely important because vaccination rollout was based on age, where the elderly were given priority. Thus, the authors of this study are highly optimistic about their research methodology, which has helped estimate total immunity accurately.
However, owing to the emergence of SARS-CoV-2 variants, immunity estimations would require further analysis and re-estimation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Desantis, MS et al. (202). Estimation of Total Immunity to SARS-CoV-2 in Texas, medRxiv, 2021.08.05.21261610; doi: https://doi.org/10.1101/2021.08.05.21261610, https://www.medrxiv.org/content/10.1101/2021.08.05.21261610v1
- Peer reviewed and published scientific report.
DeSantis, Stacia M., Luis G. León-Novelo, Michael D. Swartz, Ashraf S. Yaseen, Melissa A. Valerio-Shewmaker, Yashar Talebi, Frances A. Brito, et al. 2022. “Methodology to Estimate Natural- and Vaccine-Induced Antibodies to SARS-CoV-2 in a Large Geographic Region.” Edited by M. Kariuki Njenga. PLOS ONE 17 (9): e0273694. https://doi.org/10.1371/journal.pone.0273694. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0273694.