p>The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19), infects the human host cell via its spike antigen. Most neutralizing antibodies against SARS-CoV-2 that have been thus far discovered interact with sites on this spike glycoprotein, especially the receptor-binding domain (RBD).
 Study: Induction of cross-reactive antibody responses against the RBD domain of the spike protein of SARS-CoV-2 by commensal microbiota. Image Credit: kittipong053 / Shutterstock.com
Study: Induction of cross-reactive antibody responses against the RBD domain of the spike protein of SARS-CoV-2 by commensal microbiota. Image Credit: kittipong053 / Shutterstock.com

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Antibody response to SARS-CoV-2
The SARS-CoV-2 spike antigen mediates attachment to the angiotensin-converting enzyme 2 (ACE2) receptor that is present on the surface of human cells. The interactions between SARS-CoV-2 and ACE2 primarily occur at the RBD; therefore, this protein is a vital target for viral neutralizing antibodies. Monoclonal antibodies to the RBD have been shown to protect the host against target cell infection.
The antibody response to the virus includes IgA, IgG, and IgM, all of which are found throughout the host system. These antibodies suppress the spread of the virus from infected cells. Mucosal antibodies such as IgA2, IgA2, and IgM can also effectively neutralize viral entry at the point of contact with the host, thus preventing productive infection.
Patients with severe COVID-19 have been shown to lack SARS-CoV-2-specific IgA2 antibodies, thereby suggesting that these antibodies are protective against severe disease. However, SARS-CoV-2-naïve individuals have also been found to have detectable levels of antibodies that bind to the RBD.
The antigens of commensal microflora
One potential explanation for this observation is that prior endemic seasonal human coronavirus infection induced the production of these antibodies. However, further studies are needed to confirm this hypothesis.
Previous studies have indicated that gastrointestinal (GI) commensals induce mucosal IgA responses. These microbiota contain millions of genes and, as a result, several million antibody-binding regions. Some of these regions, which are otherwise known as epitopes, may be similar or almost identical to host proteins, which can potentially induce autoimmunity.
Other epitopes may be cross-reactive to protein antigens from other pathogenic or non-pathogenic microbes, thereby inducing immunity to these microbes upon subsequent exposure. In fact, the gp41 of human immunodeficiency virus-1 (HIV-1) is a target of cross-reactive antibodies elicited by gut microbes.
Such microbial cross-reactive immunity has been established to protect the host against many pathogens including Clostridium difficile, Pseudomonas aeruginosa, and the influenza virus. Several different mechanisms have been postulated, including toning of the innate immune system by interferon I (IFN I) production or by cross-reactive antibody production.
What were the study findings?
In the current study, the researchers identified two individuals out of 12 healthy uninfected donors who were previously unexposed to SARS-CoV-2 as identified by the lack of anti-SARS-CoV-2 IgG antibodies in their sera. Despite the lack of antibodies present within their sera, these individuals were found to have fecal IgA antibodies that were reactive to the RBD of SARS-CoV02.
An additional 10 out of 21 donors with a history of severe COVID-19 were found to contain SARS-CoV-2 RBD-specific IgA in their stool. Interestingly, the anti-RBD IgA titer negatively correlated with the age of the donor.
Upon purification of the fecal IgA antibodies, the researchers found that the neutralizing activity was low. In fact, complete inhibition of ACE2-RBD binding was not observed, even at equimolar concentrations.
Notably, the IgA antibodies from some healthy donors with anti-RBD antibodies did not suppress ACE2-RBD interactions. Thus, uninfected individuals may have neutralizing and non-neutralizing IgA antibodies to the virus. Interestingly, while healthy donors had RBD-directed IgA2 antibodies in feces, those with severe COVID-19 lacked these antibodies.
Among the two groups of healthy donors with IgA1 and IgA2 antibodies coating the intestinal mucosa, only one group had anti-RBD IgA in stool. These two groups of patients showed IgA binding to two different sets of bacteria, indicating that RBD-IgA plays a major role in commensal recognition by mucosal IgA.
The researchers also found that most of the purified neutralizing anti-RBD antibodies in this study were bound to different commensal bacteria. The most commonly recognized was Bacteroides, with others binding to Clostridia, Streptococci, Escherichia, and Bifidobacteria. The bacteria Parabacteroides and Bilophila were also bound by anti-RBD IgA and RBD-specific IgG antibodies.
Salivary IgA of high titers bound to streptococcal and bacillary species was also identified. Thus, commensal microbes express protein antigens that cross-react with some neutralizing anti-RBD antibodies.
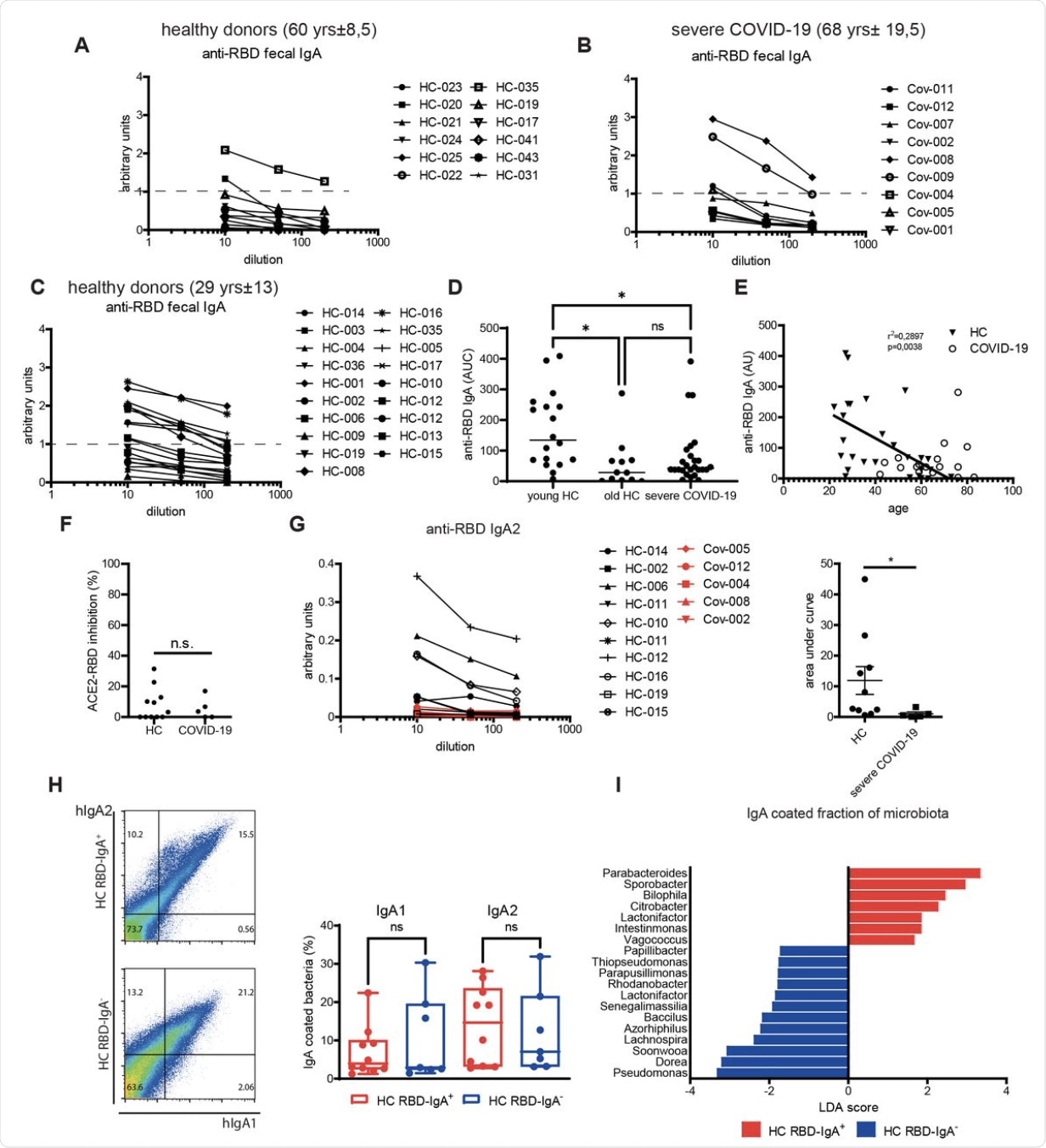
Levels of anti-RBD IgA in fecal supernatants of age-matched healthy (A) and severe COVID-19 (B) individuals. (C) Levels of anti-RBD IgA in fecal supernatants of young healthy individuals. (D) Area under the curve (AUC) values for the anti-RBD IgA ELISA measurement of the donors presented in (A-C). (E) Correlation of the levels of anti-RBD IgA with the age in healthy individuals and COVID-19 patients. Inhibition of RBD binding to ACE2 by IgA purified from feces of healthy people and severe COVID-19 patients. (G). Levels and AUC values of anti-RBD IgA2 in purified IgA fraction from healthy and severe COVID-19 individuals. (H). Representative dot plots and quantification of fecal IgA coating from healthy individuals that have anti-RBD IgA (HC RBD-IgA+) or lack anti-RBD IgA (HC RBD-IgA-). (I) Linear discriminant analysis (LDA) scores of the IgA bound bacterial fraction isolated from HC RBD-IgA+ and HC RBD-IgA-. *, p<0.05, **, p<0.01, ***, p<0.001, as calculated by unpaired t-test (F, G, H) or by Kruskal-Wallis test with Dunn’s multiple comparisons (D); ns, not significant.
Bacteria induce RBD-targeting IgA
Following these observations, the authors then aimed to understand whether these bacteria could induce a cross-reactive antibody response to the viral RBD. To this end, many of these bacteria were, in fact, found to elicit anti-RBD IgG antibodies in mice. When challenged with the bacteria orally, the production of fecal IgA directed against the RBD was also induced.
The fecal supernatants from the latter mice were also found to successfully prevent ACE2-RBD binding. In fact, two bacterial species including B. pseudocatenulatum and S. salivarius elicited antibodies to the receptor-binding motif (RBM) of the RBD, which accounted for this inhibition.
In rabbits, the anti-RBD and the human IgA antibody CV07-200 showed overlapping peptide recognition patterns, with similar peptide sequences within the RBM.
“These data show that oral supplementation with S. salivarius K12 and B. pseudocatenulatum can induce antibodies cross-reactive against the RBM motif of the spike protein of SARS-CoV-2.”
The commensal population of the oral cavity in severe COVID-19 is quite different from that of healthy subjects, those with mild COVID-19, or those with symptoms of the flu. In healthy individuals, the number of bacterial species is significantly reduced, especially the Veillonella and Streptococcus genera, while sparing Bifidobacteria genera.
However, genera like Enterococcus, Staphylococcus, and Escherichia/Shigella were increased. This difference could not be the result of antibiotic treatment, since all patients in the group had not received antibiotics.
Intestinal microbiota also reflected the same changes. Thus, severe COVID-19 is linked to the growth of opportunistic bacterial genera like Enterococci and Staphylococci, with others being lost or reduced.
Mucosal infection with SARS-CoV-2 induces inflammation and sometimes fatal COVID-19. The risk of severe COVID-19 is considerably higher in individuals with autoantibodies against IFN I, certain specific genes, older age, as well as those with underlying health conditions like obesity or diabetes.
Cross-reactive but low-avidity T-cells generated by earlier microbes but specific for SARS-CoV-2 may not only fail to neutralize the virus but instead enhance SARS-CoV-2 binding and disease severity. In this case, however, uninfected individuals showed high titers of pre-existing mucosal IgA targeting the viral RBD that were capable of neutralizing the virus.
What are the implications?
The GI microbiota helps to protect the host by reducing ACE2 receptor expression and inducing IFN I responses in a tonic manner. These microorganisms also modulate antibody switching to IgA via transforming growth factor β1 (TGF-β1), both locally and systemically.
In the current study, the investigators identified the specific oral bacteria that have cross-reactive antigenic epitopes on their surfaces that are capable of inducing cross-reactive antibodies to SARS-CoV-2 RBD. This allows these bacteria to both be recognized by anti-RBD antibodies that are elicited by the virus, and capable of inducing anti-RBD neutralizing antibodies against the virus.
Taken together, healthy donors with these bacteria appear to have mucosal IgA that recognizes SARS-CoV-2 RBD and inhibits RBD-ACE2 binding, despite their negative history of infection with the virus. Further studies will be required to understand the underlying mechanism of cross-reactivity and the direction of causality for the altered microbiota seen in acute severe COVID-19.
In immunocompromised patients with poor immune responses to COVID-19 vaccination, the researchers propose: “Bacteria supplementation, in particular with S. Salivarius K12, may enhance the titers of anti-RBD IgA antibodies at the mucosal surfaces, prophylactically or therapeutically, or even in the context of vaccination.”
However, this requires research to understand whether such antibodies will protect against SARS-CoV-2 infection or severe COVID-19.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.