The ongoing coronavirus disease 2019 (COVID-19) pandemic has severely impacted the world’s healthcare system and economy. COVID-19, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected over 214 million and claimed the lives of almost 4.5 million worldwide. Individuals can be protected from SARS-CoV-2 via immunity that is induced by either a previous SARS-CoV-2 infection or vaccination.
 Study: Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Image Credit: Toa55 / Shutterstock.com
Study: Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Image Credit: Toa55 / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Previous studies have found that COVID-19 recovered individuals are approximately 90% protected from SARS-CoV-2 reinfection, while the efficacy of the available vaccines has been reported to be 50-95%. Several studies have highlighted a decrease in both the memory B-cell humoral and spike-specific CD4+ cellular immune responses to SARS-CoV-2 over time. This reduction in immune responses against SARS-CoV-2 has challenged scientists fighting hard to contain the pandemic.
Soon after Israel began its large-scale vaccination campaign, this nation was able to quickly vaccinate a majority of the adult population. As of May 26, 2021, Israel reported that more than 5 million people out of its total population of 9.3 million had received both doses of the Pfizer-BioNTech vaccine.
Despite these efforts, a recent surge in COVID-19 cases in Israel has been reported. Scientists believe it is essential to understand the main cause of such resurgence and the extent to which the highly infectious Delta variant of SARS-CoV-2 has contributed to this surge. Additional explanations for this surge in cases could be a lower efficacy of the available vaccine against the Delta variant and/or the fading of anti-SARS-CoV-2 antibodies in vaccinated individuals.
Taken together, there remains a lack of real-world data associated with the time evolution of SARS-CoV-2 antibodies after COVID-19 vaccination and after the SARS-CoV-2 infection.
A new study
A new study published on the medRxiv* preprint server focused on the decrease in antibody titers following two doses of BNT162b2 vaccine or SARS-CoV-2 natural infection in Israel. This large-scale study analyzed the kinetics of SARS-CoV-2 immunoglobulin G (IgG) antibodies after the completion of vaccination with BNT162b2 vaccine or SARS-CoV-2 infection unvaccinated individuals.
In this study, the antibody titers were measured from January 31, 2021 to July 31, 2021, in two mutually exclusive groups. Whereas the first group included individuals who received two doses of the BNT162b2 vaccine and had no history of previous COVID-19 infection, the second group included individuals who recovered from SARS-CoV-2 infection and did not receive the vaccine.
Study findings
The authors of the current study have estimated antibody titers among a large number of individuals. The study included 2,653 individuals who received two doses of the BNT162b2 vaccine during the study period and 4,361 convalescent patients.
Scientists observed higher SARS-CoV-2 IgG antibody titers in individuals who received both the vaccine doses as compared to convalescent individuals. Interestingly, the antibody titers in the vaccinated candidates decreased by up to 40% in the subsequent months. However, such a rapid decrease was not observed in the case of convalescent subjects, where the antibody titer decreased by less than 5% per month.
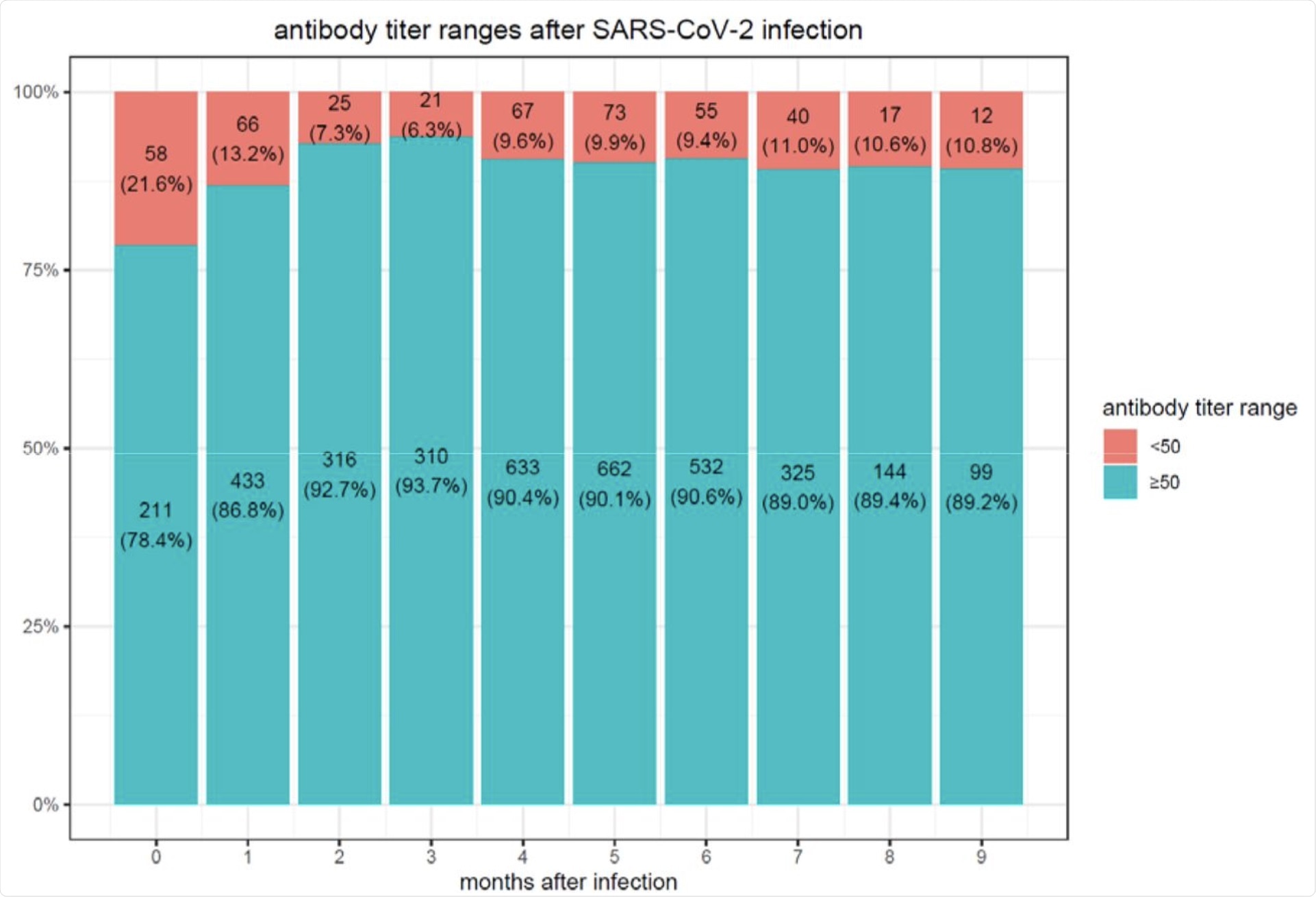
After six months, 16.1% of individuals who received the BNT162b2 vaccine showed a decrease in antibody levels below the seropositivity threshold of <50 AU/mL. Comparatively, only 10.8% of SARS-CoV-2 convalescent patients were found to have a decline in the antibody levels below the <50 AU/mL threshold 9 months after infection.
A prior study conducted on individuals who had received two doses of the BNT162b2 vaccine found that these individuals contracted SARS-CoV-2 infection after 146 days of receiving the second vaccine dose. In this case, COVID-19 infection was reported in all age groups. However, the incidence of SARS-CoV-2 was much higher among patients around 60 years of age.
The researchers of this study opined that the lowering of SARS-CoV-2 IgG antibodies may have increased the infection rate in this age group. They further proposed that a third vaccine booster dose could increase the antibody titer and thereby provide further protection to this vulnerable group.
Strength and limitations
The main strength of this study is that it has included a large cohort of vaccinated individuals and patients who had recovered from SARS-CoV-2. The decline in the antibody titers of vaccinated individuals is much steeper compared to convalescent individuals.
The observational design of the study excluded confounding factors. Another limitation is that even though the authors have adjusted the results for known factors, the production and decay of antibodies could be affected by several other factors.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Israel, I., Shenhar, Y., Green, I. et al. (2021) Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. medRxiv. doi:10.1101/2021.08.19.21262111. https://www.medrxiv.org/content/10.1101/2021.08.19.21262111v1.
- Peer reviewed and published scientific report.
Israel, Ariel, Yotam Shenhar, Ilan Green, Eugene Merzon, Avivit Golan-Cohen, Alejandro A. Schäffer, Eytan Ruppin, Shlomo Vinker, and Eli Magen. 2021. “Large-Scale Study of Antibody Titer Decay Following BNT162b2 MRNA Vaccine or SARS-CoV-2 Infection.” Vaccines 10 (1): 64. https://doi.org/10.3390/vaccines10010064. https://www.mdpi.com/2076-393X/10/1/64.