Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogenesis is a multistep process that involves the infection of angiotensin-converting enzyme 2 (ACE2)-expressing lung epithelial cells followed by uncontrolled viral replication, which leads to cell lysis and the release of damage-associated molecular patterns (DAMPs).
When the neighboring cells recognize these molecules, they produce a pro-inflammatory environment by releasing cytokines such as Interleukin 6 (IL-6) and Interleukin-10 (IL-10), which recruit and activate macrophages, monocytes, and T cells (also called T lymphocytes).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In the case of severe COVID-19 patients, this pro-inflammatory feedback loop leads to a harmful response that causes structural damage to the lung. The cytokine storm that results can cause acute respiratory distress syndrome (ARDS) and organ failure. Although DAMPs can drive systemic inflammation in many settings, it is unclear if it occurs in COVID-19.
“Aberrant and rapid increase in a broad-spectrum of pro-inflammatory cytokines, known as a cytokine storm, plays a central role in the pathogenesis of ARDS and other severe complications of SARS-CoV-2 infection.”
Evaluating the impact of CD24Fc treatment in severe COVID-19 patients
The therapeutic efficacy of anti-viral and anti-inflammatory agents against SARS-CoV-2 has been shown to be limited. Soluble CD24 (CD24Fc), a highly glycosylated protein, has been shown to diminish the overall inflammatory response induced by DAMPs. A recent randomized phase III trial conducted by researchers at the Ohio State University, Columbus, OH, US, evaluated the impact of CD24Fc in severe COVID-19 patients and showed promising clinical efficacy. This study is available as a preprint on the medRxiv* server.
The researchers analyzed peripheral blood samples collected from 22 patients enrolled in the SAC-COVID trial (NCT04317040). The samples were collected before and at many points after CD24Fc or placebo treatment. They performed high-dimensional spectral flow cytometry analysis and assessed cytokine levels to determine the immunological impact of treatment with CD24Fc on COVID-19 patients.
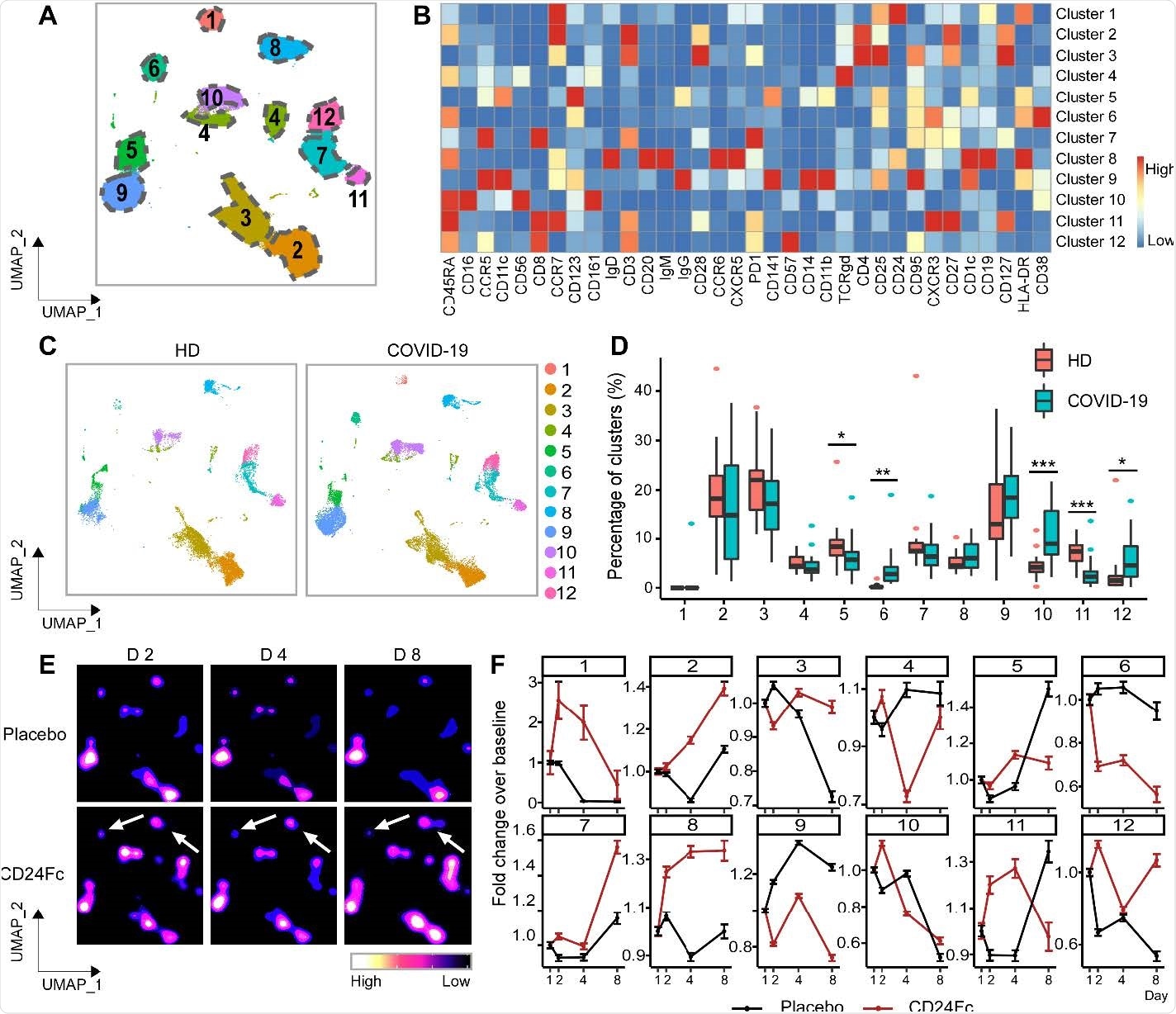
Population dynamics of peripheral blood mononuclear cells from healthy donors vs. patients with COVID-19 treated with placebo or CD24Fc. A total of 1,306,473 PBMCs from HD (n=17) and COVID-19 patients (n=22) were clustered using an unbiased multivariate t-mixture model, which identified 12 sub-clusters that reflect statistically distinct cell states. Visualization of the relative similarity of each cell and cell cluster on the two-dimensional UMAP space with a 10% downsampling (Panel A). Cluster-by-marker heatmap characterizing the expression patterns of individual clusters (Panel B). UMAP dot plots (Panel C) and cluster frequencies (Panel D) of HD vs. baseline COVID-19 patient samples (cluster 5, p=0.03; cluster 6, p=0.001; cluster 10, p<0.001; cluster 11, p<0.001). Contour plots representing the density of cells throughout regions of the UMAP space from COVID-19 patients D2, D4, and D8 after CD24Fc vs. placebo treatment (Panel E, white arrows indicate visual changes between CD24Fc vs. placebo contour plots). Selected cluster population dynamics as fold change over baseline for each group over time (Panel F) (D2: placebo n=12, CD24Fc n=10; D4: placebo n=11, CD24Fc n=9; D8: placebo n=4, CD24Fc n=3). The p-value was calculated using the Kenward-Roger method. *, p<0.05; **, p<0.01; ***, p<0.001.
Patient characteristics from the CD24Fc vs. placebo groups were matched clinically so as to compare results without confounding factors. With the help of high-content spectral flow cytometry, the researchers found systemic hyper-activation of multiple cell compartments in the placebo group, including CD4+ T cells, CD8+ T cells, and CD56+ NK cells in patients having untreated COVID-19.
“In addition to the two therapeutic targets, we also identified two cytokines that were significantly downregulated after CD24Fc treatment: IL-10 and IL-15.”
In comparison, CD24Fc-treated patient samples showed dampened systemic inflammation, with a return to homeostasis in NK and T cells within days. In addition, a single CD24Fc dose significantly weakened systemic IL-10 and IL-15 cytokines and dampened the coexpression and networking among inflammatory cytokines associated with severe COVID-19.
“Our clinical and immunological data support further development of CD24Fc as a novel therapeutic against severe COVID-19.”
CD24Fc treatment leads to accelerated recovery in severe COVID-19 patients compared to placebo treatment
The severe COVID-19 patients in this study showed accelerated recovery after CD24Fc treatment compared to placebo treatment. CD24Fc was mostly well tolerated, decreased disease progression, and shortened length of hospital stay in pts with COVID-19. Based on SARS-CoV-2’s proposed mechanism of action and pathophysiology, the authors hypothesized that CD24Fc decreased the hyperactive systemic immune responses in patients which led to an accelerated return to homeostasis.
In conclusion, the study results offer unique insights into the immunological impact of SARS-CoV-2 that complement the clinical findings of the SAC-COVID trial. Furthermore, these findings strongly support further investigation of CD24Fc for many inflammatory conditions, including COVID-19.
“Our unique cytokine centrality analysis and cellular activation index also warrant further study as a prognostic tool for guiding therapy in COVID-19 and other systemic inflammatory conditions.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Immunological Insights Into the Therapeutic Roles of CD24Fc Against Severe COVID-19, No-Joon Song, Carter Allen, Anna E. Vilgelm, Brian P. Riesenberg, Kevin P. Weller, Kelsi Reynolds, Karthik B. Chakravarthy, Amrendra Kumar, Aastha Khatiwada, Zequn Sun, Anjun Ma, Yuzhou Chang, Mohamed Yusuf, Anqi Li, Cong Zeng, John P. Evans, Donna Bucci, Manuja Gunasena, Menglin Xu, Namal P.M. Liyanage, Chelsea Bolyard, Maria Velegraki, Shan-Lu Liu, Qin Ma, Martin Devenport, Yang Liu, Pan Zheng, Carlos D. Malvestutto, Dongjun Chung, Zihai Li, medRxiv, 2021.08.18.21262258; doi: https://doi.org/10.1101/2021.08.18.21262258, https://www.medrxiv.org/content/10.1101/2021.08.18.21262258v1
- Peer reviewed and published scientific report.
Song, No-Joon, Carter Allen, Anna E. Vilgelm, Brian P. Riesenberg, Kevin P. Weller, Kelsi Reynolds, Karthik B. Chakravarthy, et al. 2022. “Treatment with Soluble CD24 Attenuates COVID-19-Associated Systemic Immunopathology.” Journal of Hematology & Oncology 15 (1). https://doi.org/10.1186/s13045-021-01222-y. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-021-01222-y.