Scientists and pharmaceutical companies have successfully generated vaccines to fight the coronavirus disease (COVID-19) pandemic. Most of these vaccines are very effective in combating the disease.
However, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus that has a propensity to mutate often. This may result in the vaccines against COVID-19 becoming ineffective over time as new strains evolve. Moreover, the immunity against the virus was found to decrease over time, in people who have had prior COVID-19 infection and in the vaccinated population.
The immunity against COVID-19 is conferred by the neutralizing antibodies (NAbs), both occurring during the post-infection period and upon vaccination. Unfortunately, these antibodies are not maintained at stable levels in the body and tend to decay over time. This raises a need for subsequent booster doses for long-term protection against the virus.
Presently, there is a need to develop improved versions of COVID-19 vaccines that are effective against the SARS-CoV-2 variants of concern (VOC) and can confer long-term protection. Considering the need of the hour, scientists from Australia, the USA, and Switzerland have generated a new COVID-19 vaccine CoVac-II and evaluated its effect against SARS-CoV-2 variants. The findings from this study are published on the bioRxiv* preprint server while awaiting peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
CoVac-II a new COVID-19 vaccine candidate
Toll-like receptor (TLR)-7 and TLR-8 agonists have earlier been reported to contribute to the immune response against viruses. Alhydroxiquim-II (AHQ-II), a TLR– 7/8 agonist, has been effectively used in COVAXIN (COVID-19 vaccine). Spike proteins are present on the surface of SARS-CoV-2 and are essential for viral pathogenesis by facilitating viral entry into host cells. The new candidate vaccine CoVac-II was generated as a combination of AHQ-II and SARS-CoV-2 spike protein to provoke an immune response in the host.
Evaluation of CoVac-II
CoVac – II was administered to mice, and its immunogenicity was evaluated and compared against controls; spike protein alone (SPK) or spike protein combined with adjuvant Alhydrogel (SPKalum).
CoVac-II and NAbs production
At two weeks after the first dose of CoVac-II was administered to mice, it resulted in elevated levels of NAbs in their plasma. The levels of NAbs remained stable without decaying, reaching their peak at 252 days after vaccination. CoVac -II performed better than controls SPK or SPKalum. The adjuvant AHQ-II, when administered alone to mice, increased NAb levels by nearly 1,000 fold. The immunogenic properties of AHQ-II seem to be better than other COVID-19 vaccine adjuvants like Matrix-M and AS03.
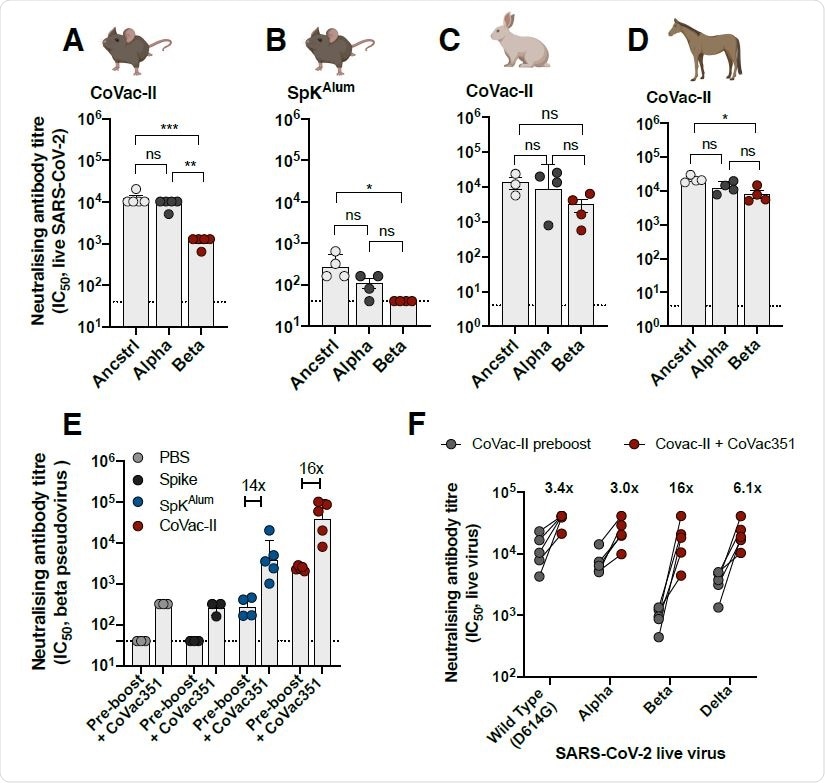
Alhydroxiquim-II-adjuvanted vaccines afford cross-species neutralization of variants of concern, that is augmented by a variant-specific booster vaccine. Mice (n=4 to 5) were vaccinated as in Figure 1 and 3 weeks post-vaccination, plasma from CoVac-II (A) or SpKAlum (B) tested for neutralizing activity against live SARS-CoV-2 infection of Vero E6 cells (ancstrl=ancestral virus). C. Rabbits (n=3) were immunized i.m. twice with CoVac-II (ancestral 5 μg spike/200 μg Alhydrogel) and NAb titers against live SARS-CoV-2 viruses determined. D. Horses (n=3) were immunized i.m. twice with CoVac-II (ancestral 20 μg spike/500 μg Alhydrogel) and NAb titers against live SARS-CoV-2 viruses determined. E. Mice vaccinated 250 days previously were boosted with a single dose of CoVac351 (5 μg Beta spike/100 μg Alhydroxiquim-II) and NAb titers against Beta spike-pseudovirus determined at one-week post-boost. Data presented as geometric mean ± geometric SD. F. Plasma from CoVac351-boosted mice was tested for neutralizing activity against live SARS362 CoV-2 infection of Vero E6 cells. The dotted line shows the limit of detection. Significant differences between groups were determined by one-way ANOVA.
CoVac-II and T cell immunity
T cells and substances like Interferon (IFN) -γ, Tumor necrosis factor (TNF)-α, and Interleukin (IL)-2 play a role in the immune response of our body. CD4 (cluster of designation 4)+- T cells secreting Interferon (IFN)-γ, Tumor necrosis factor (TNF)-α, and Interleukin (IL)-2 simultaneously are termed as multifunctional CD4+-T cells. The levels of multifunctional CD4+-T cells that are provoked in response to the spike protein in CoVac-II were measured. It was found to be significantly elevated in mice vaccinated with CoVac-II compared to mice that received controls.
The levels of antigen-specific B cells and T follicular helper (Tfh) cells were also significantly elevated in CoVac-II vaccinated mice.
Effect of CoVac-II on SARS-CoV-2 variants of concern (VOC)
The neutralizing effect of the antibodies produced in mice in response to CoVac-II was tested against ancestral (Wuhan) COVID-19 strain and Alpha and Beta variants. It was found that the NAbs were effective against all the variants tested, and it was also observed that their effect was slightly reduced against the Beta variant. Cross-species neutralization of the VOC tested was also observed from NAbs obtained from CoVac-II vaccinated rabbits and horses.
The current vaccines available against COVID-19 were found to exhibit low efficacy against the Beta variants. A vaccine was generated by combining the spike protein of the beta variant with AHQ-II (CoVac-351) and its effect as a booster dose was evaluated. Mice that had been vaccinated with CoVac-II, 8 months before were given a booster dose of CoVac-351. Mice vaccinated with CoVac-351 showed elevated levels of multifunctional Th1 CD4+ cells. Further, it was found that NAbs from the plasma of these mice were effective against Alpha, Beta, and Delta variants. The NAbs from mice that received the booster vaccines performed better than the NAbs obtained from mice that did not receive the booster dose against all strains tested. This effect was significantly higher against the Beta variant. Further, the NAbs seem to perform well against the Delta variants as well, with only a slight reduction in effect compared to the ancestral strain.
Promising vaccine candidate against COVID-19
The novel candidate vaccine CoVac-II is a promising vaccine candidate against COVID-19 because:
- It provokes an effective immune response in vaccinated mice by elevating NAb levels which are also sustained for a long duration of 8 months after vaccination.
- It is effective against VOCs and its effect can be enhanced by an added booster dose of vaccine containing variant-specific spike protein.
- It has been found to function better than other COVID-19 vaccines (currently in clinical use) tested in the same animal models used in this study.
- It has a good safety profile, immunogenicity, and its large scale manufacturing can be performed easily

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Sources:
Journal references:
- Preliminary scientific report.
Neutralising antibodies against the SARS-CoV-2 Delta variant induced by Alhydroxyquim-II-adjuvanted trimeric spike antigens, Claudio Counoupas, Paco Pino, Alberto O. Stella, Caroline Ashley, Hannah Lukeman, Nayan D. Bhattacharyya, Takuya Tada, Stephanie Anchisi, Charles Metayer, Jacopo Martinis, Anupriya Aggarwal, Belinda M. Dcosta, Joeri Kint, Maria J Wurm, Nathaniel R. Landau, Megan Steain, Stuart G Turville, Florian M Wurm, Sunil A. David, James A. Triccas, bioRxiv, 2021.08.18.456891; doi: https://doi.org/10.1101/2021.08.18.456891, https://www.biorxiv.org/content/10.1101/2021.08.18.456891v1
- Peer reviewed and published scientific report.
Counoupas, Claudio, Paco Pino, Alberto O. Stella, Caroline Ashley, Hannah Lukeman, Nayan D. Bhattacharyya, Takuya Tada, et al. 2022. “High-Titer Neutralizing Antibodies against the SARS-CoV-2 Delta Variant Induced by Alhydroxyquim-II-Adjuvanted Trimeric Spike Antigens.” Edited by Angela Bordin. Microbiology Spectrum 10 (1). https://doi.org/10.1128/spectrum.01695-21. https://journals.asm.org/doi/10.1128/spectrum.01695-21.