Researchers in Israel have conducted a study showing that vaccinating pregnant women against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) early on in the third trimester may maximize transplacental antibody transfer, potentially helping to prevent severe coronavirus disease 2019 (COVID-19) during infancy.
The team – from Hadassah-Hebrew University Medical Center in Jerusalem and the University of Haifa –found that maternal immunization with the Pfizer-BioNTech’s COVID-19 vaccine during the early-third trimester (27-31 weeks) was associated with higher neonatal levels of anti-SARS-CoV-2 antibodies and serum neutralizing activity than immunization during the late-third trimester (32-36 weeks).
Amihai Rottenstreich and colleagues say that since the strategy of maternal SARS-CoV-2 immunization is gaining support, further scientific evidence is urgently needed to inform the ideal timing of vaccination that would provide mothers and neonates with the highest clinical protection against COVID-19.
“The current study results indicate that early-third trimester immunization has the potential to maximize maternofetal transplacental antibody transfer, thereby affording adequate seroprotection during early infancy,” they write.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Pregnant women and infants are at an increased risk of severe SARS-CoV-2 infection
Pregnant women and their infants, particularly neonates, are at an increased risk of developing severe SARS-CoV-2 infection. Pregnant women are more likely to be admitted to intensive care and to require invasive ventilation than nonpregnant women and maternal infection has been associated with adverse perinatal outcomes, including preterm delivery and stillbirth. Infants are also at a significantly higher risk of severe disease than older children.
Pfizer-BioNTech’s BNT162b2 vaccine has been shown to elicit a robust immune response among pregnant women, and recent studies have shown that antenatal vaccination may lead to transplacental transfer of anti-SARS-CoV-2 antibodies.
“As children, including young infants, are currently not eligible for SARS-CoV-2 vaccination, offering neonatal seroprotection in the early, vulnerable stages of life through maternal immunization is of paramount importance,” says Rottenstreich and colleagues. “This principle is well-established for the prevention of other potentially life-threatening respiratory infections such as pertussis and influenza.”
However, the team adds that defining the optimal timing of this maternal immunization is crucial to maximizing maternofetal antibody transfer and optimizing protection during infancy.
What did the researchers do?
The researchers conducted a prospective study of pregnant women admitted for delivery at Hadassah Medical Center between February and April 2021. The team assessed transplacental antibody transfer in 171 women (median age 31 years) following antenatal immunization with the BNT162b2 vaccine, with the first dose given between 27 and 36 weeks gestation.
Eighty-three (48.5%) of the women were immunized during the early-third trimester (1st dose at 27-31 weeks), and 88 (51.5%) were immunized during the late-third trimester (1st dose at 32-36 weeks).
Maternal and cord blood sera were collected following term delivery and tested for SARS-CoV-2 spike protein (S) and receptor-binding domain (RBD)-specific immunoglobulin G (IgG) levels, as well as neutralizing potency. The spike protein mediates the initial stage of the SARS-CoV-2 infection process when its RBD attaches to the host cell receptor angiotensin-converting enzyme 2.
Since the first vaccine dose and delivery, the median time was 71 days among those immunized in the early-third trimester and 41 days among those immunized in the late-third trimester.
What did the study find?
Serum samples from all 171 mother-infant pairs were positive for anti S- and anti-RBD-specific IgG, with a positive correlation observed between maternal and neonatal cord blood concentrations.
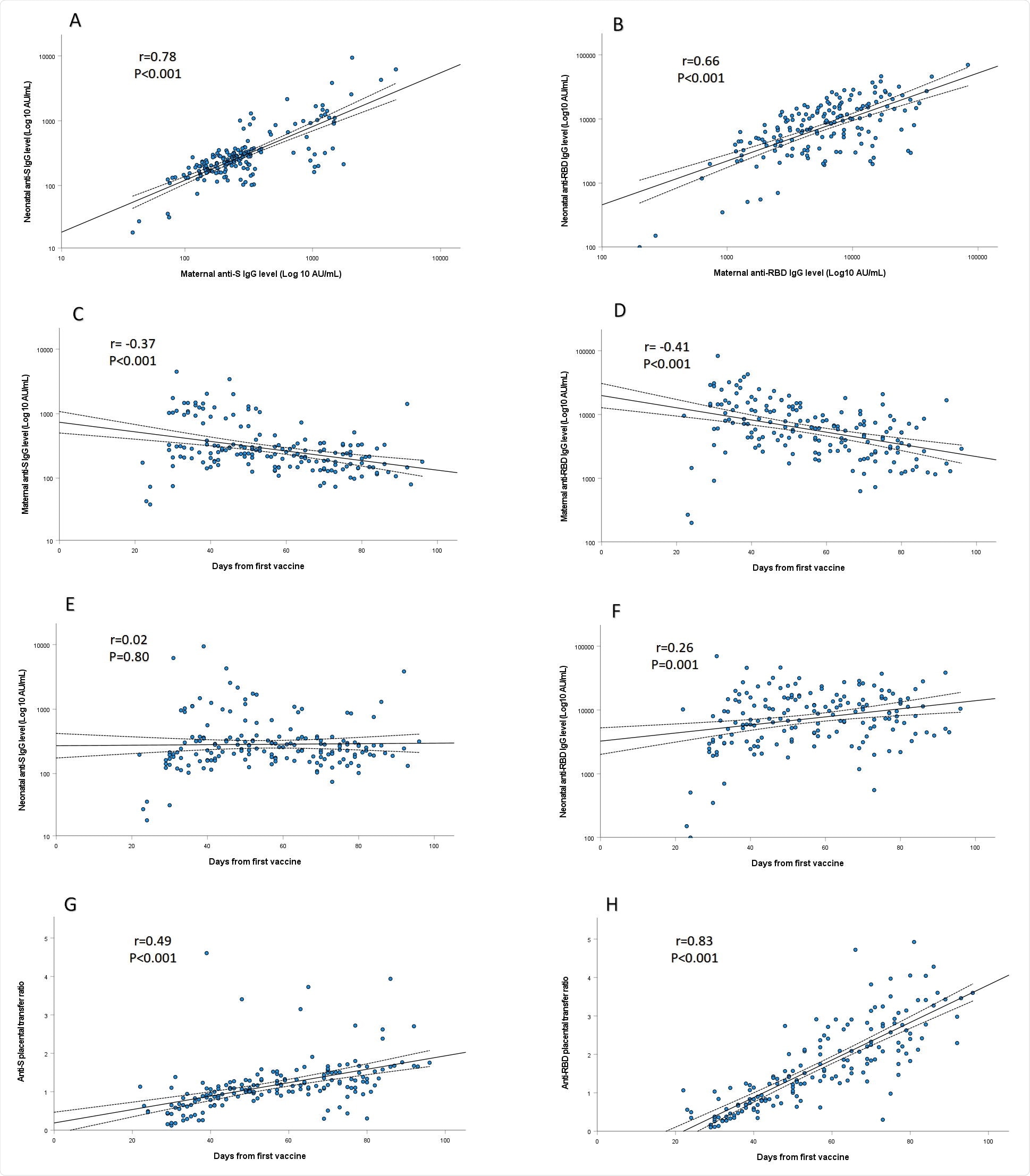
SARS-CoV-2 anti-S (A) and anti-RBD-specific (B) IgG levels in maternal sera were positively correlated to their respective concentrations in cord blood (r=□0.78; P□<0.001 and r=□0.66; P□<0.001, respectively). Maternal anti-S (C) and anti-RBD-specific (D) IgG concentrations were negatively associated with the time lapsed since immunization (r=□-0.37; P□<0.001 and r=□-0.41; P□<0.001, respectively). Neonatal concentrations of anti-S (E) IgG antibodies did not differ in relation to maternal third trimester immunization timing (P=0.80). Anti-RBD-specific (F) IgG concentrations in neonatal sera were positively correlated with increasing time since vaccination (r=□0.26; P=0.001). Placental transfer ratios of anti-S (G) and anti-RBD-specific (H) IgG were directly associated with longer duration since immunization (r=□0.49; P□<0.001 and r=□0.83; P□<0.001, respectively). Correlations, as well as correspondent R and P values were calculated by Pearson ‘s test, as shown in each panel. The dotted lines are the 95% confidence intervals.
The anti-RBD-specific IgG concentrations in neonatal sera were significantly higher among infants born to mothers vaccinated in the early-third versus late-third trimester (median 9620 versus 6697 AU/mL) and were positively correlated with increasing time since vaccination.
The median placental transfer ratios of anti-S and anti-RBD specific IgG were also significantly higher following early-third versus late-third trimester immunization (anti-S ratio:1.3 versus 0.9; anti-RBD ratio:2.3 versus 0.7) and were directly associated with longer duration since immunization.
Multivariate analysis revealed that the only predictor of anti-RBD-specific IgG neonatal concentrations and placental transfer ratio was the duration of time since first vaccination, with no association identified for any other maternal, pregnancy or delivery characteristics.
The placental transfer ratio of SARS-CoV-2 neutralizing antibodies was also significantly higher following early-third versus late-third trimester immunization (1.9 versus 0.8) and was positively associated with increasing time since vaccination.
What did the authors conclude?
Rottenstreich and colleagues say the findings may support the role of vaccination early in the third trimester to optimize maternofetal antibody transfer and neonatal seroprotection.
The researchers also say that the results are in accordance with previous reports evaluating the effect of maternal pertussis and influenza immunization, which showed augmented transplacental transfer and neonatal antibody levels along with improved clinical outcomes following early-third trimester vaccination.
However, “while encouraging, future studies should evaluate the kinetics and durability of these passively acquired antibodies in the offspring and their protective effect against with clinical COVID-19- related outcomes,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Rottenstreich A, et al. Early versus late third trimester maternal SARS-CoV-2 BNT162b2 mRNA immunization maximizes transplacental antibody transfer and neonatal neutralizing antibody levels, medRxiv, 2021. doi: https://doi.org/10.1101/2021.08.30.21262875, https://www.medrxiv.org/content/10.1101/2021.08.30.21262875v1
- Peer reviewed and published scientific report.
Rottenstreich, Amihai, Gila Zarbiv, Esther Oiknine-Djian, Olesya Vorontsov, Roy Zigron, Geffen Kleinstern, Dana G. Wolf, and Shay Porat. 2021. “Timing of SARS-CoV-2 Vaccination during the Third Trimester of Pregnancy and Transplacental Antibody Transfer: A Prospective Cohort Study.” Clinical Microbiology and Infection, November. https://doi.org/10.1016/j.cmi.2021.10.003. https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00601-7/fulltext.