Scientists from the UK and USA have developed an antifouling nanocomposite-coated multiplexed electrochemical sensor platform that can simultaneously detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA and virus-specific antibodies. The platform has shown 100% accuracy in detecting viral RNA and IgG-specific anti-SARS-CoV-2 antibodies. The study is currently available on the medRxiv* preprint server.
Large-scale administration of coronavirus disease 2019 (COVID-19) vaccine together with rapid and accurate detection of acute SARS-CoV-2 infection are the key measures to be taken to control the pandemic trajectory. While reverse transcription-polymerase chain reaction (RT-PCR)-based detection of viral RNA in respiratory samples is considered the most accurate method for COVID-19 diagnosis, serological antibody testing is the most appropriate platform to detect previous SARS-CoV-2 infection as well as vaccine efficacy.
In the current study, scientists have developed an electrochemical sensor-based portable diagnostic device that can detect viral RNA and virus-specific antibodies simultaneously.
Electrochemical sensor platform
The scientists developed a multiplexed electrochemical sensor platform that combines CRISPR/Cas-based molecular detection of SARS-CoV-2 nucleic acids (viral RNA) and multiplexed serological detection of antibodies against three SARS-CoV-2 proteins, including spike receptor-binding domain (RBD), spike S1 subunit, and nucleocapsid protein.
Schematic and representative raw cyclic voltammetry data of the multiplexed serology assay. (a) Schematic illustrating the multiplexed electrochemical serological assay to assess host antibody responses on electrodes functionalized with SARS-CoV-2 antigens. Host antibodies bind to the SARS-CoV-2 antigens immobilized on the chips. Subsequently, biotinylated anti-human IgG secondary antibodies bind, followed by poly HRP-streptavidin binding and TMB precipitation on the chips. (b-e) Typical cyclic voltammograms for the four different electrodes that target host antibodies against (b) Spike 1 subunit (S1), (c) Spike 1-receptor binding domain (S1-RBD), (d) nucleocapsid (N), and (e) BSA negative control with positive (red, blue) and negative (orange and black) samples for IgG and IgM, respectively.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Although electrochemical sensors integrated with biological probes are highly cost-effective and ultra-sensitive platforms for simultaneously detecting viral nucleic acids and proteins, one major disadvantage is the accumulation of waste materials of biological samples on the sensor surface. This biofouling can significantly reduce the sensitivity and specificity of the sensor by altering surface chemistry and reducing signal strength. To overcome this limitation, the scientists coated the sensor (gold chip) with an antifouling nanocomposite composed of glutaraldehyde cross-linked bovine serum albumin covered with conductive reduced graphene oxide. After surface coating, gold chips were activated by chemical compounds, and capture probes were spotted on top of the electrode area.
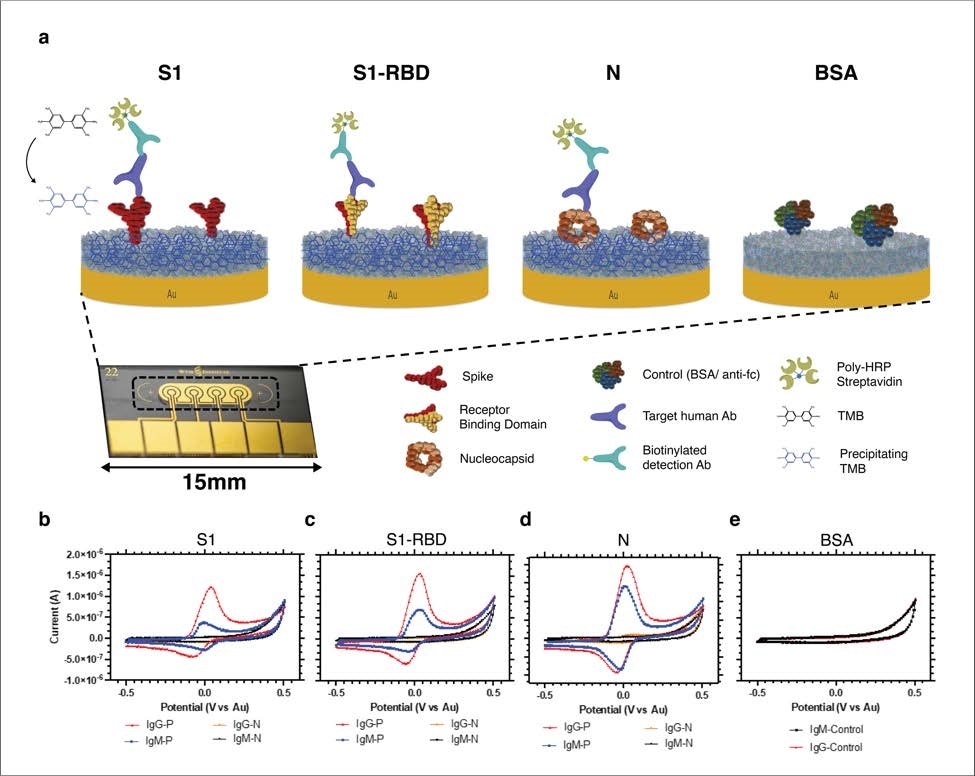
Schematic and representative raw cyclic voltammetry data of the multiplexed serology assay. (a) Schematic illustrating the multiplexed electrochemical serological assay to assess host antibody responses on electrodes functionalized with SARS-CoV-2 antigens. Host antibodies bind to the SARS-CoV-2 antigens immobilized on the chips. Subsequently, biotinylated anti-human IgG secondary antibodies bind, followed by poly HRP-streptavidin binding and TMB precipitation on the chips. (b-e) Typical cyclic voltammograms for the four different electrodes that target host antibodies against (b) Spike 1 subunit (S1), (c) Spike 1-receptor binding domain (S1-RBD), (d) nucleocapsid (N), and (e) BSA negative control with positive (red, blue) and negative (orange and black) samples for IgG and IgM, respectively.
They integrated the CRISPR/Cas-based assay into the electrochemical sensor by partially hybridizing a biotinylated single-stranded DNA reporter probe to peptide nucleic acid capture probes, which were immobilized on the sensor surface.
To develop the multiplexed serological platform, they initially performed an enzyme-linked immunosorbent assay (ELISA) to optimize three primary viral antigens that induce antibodies during SARS-CoV-2 infection. These antigens were spike RBD, spike S1 subunit, and nucleocapsid protein. Afterward, they functionalized each gold electrode individually with these optimized viral antigens to develop the multiplexed serology electrochemical sensor platform.
Important observations
The scientists conducted a series of experiments to validate the diagnostic potency and accuracy of the multiplexed electrochemical sensor platform.
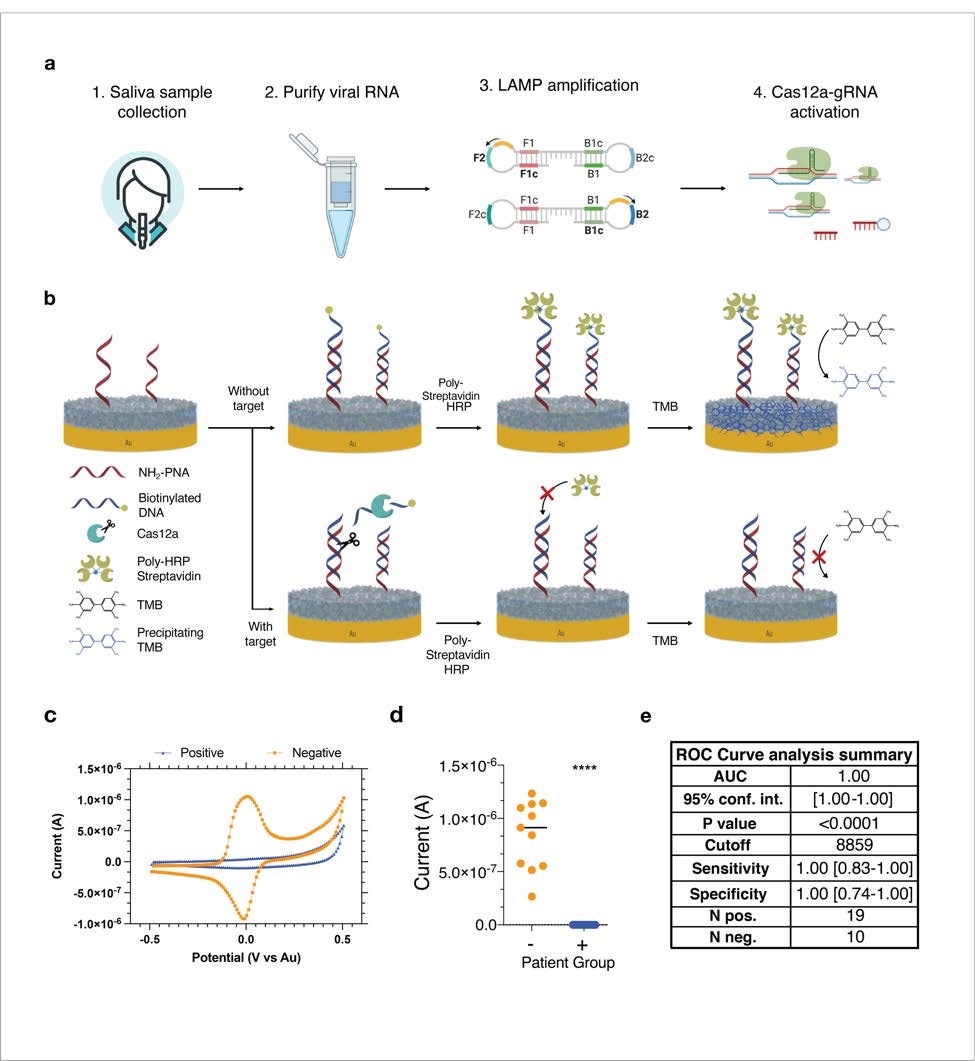
The RNA extracted from 19 saliva samples of SARS-CoV-2-infected patients was used for the CRISPR/Cas-based assay. As controls, the RNA was extracted from eleven RT-PCR-negative saliva samples. The findings revealed that the CRISPR/Cas-based platform detects viral RNA with 100% accuracy and that there is a significant correlation between CRISPR/Cas-based and RT-PCR-based detection of viral RNA.
Similarly, testing of 58 virus-positive plasma samples and 54 virus-negative plasma samples using the serological electrochemical sensor platform showed 100% sensitivity and specificity against IgG-specific anti-SARS-CoV-2 antibodies and 94% specificity and 82% sensitivity for IgM-specific anti-SARS-CoV-2 antibodies. The testing accuracy was highest for the anti-spike RBD antibodies, followed by anti-nucleocapsid and anti-S1 antibodies.
Validation of multiplexed CRISPR-based assay and serological assay
The scientists modified each electrode with one of the three tested antigens to detect viral RNA and antibodies simultaneously in a single chip. They conducted validation experiments using clinical saliva samples as it is a rich source of both viral RNA and antiviral host antibodies.
They spiked the heat-inactivated saliva samples with SARS-CoV-2-infected plasma samples to stimulated the ratio of saliva IgG antibodies compared to human serum. Then, they divided each plasma-spiked saliva sample into two portions for simultaneous detection of viral RNA and antiviral antibodies.
The findings revealed that a single electrochemical sensor chip with multiple electrodes has 100% sensitivity and specificity to detect viral RNA and host antibodies within a short period of time (30 minutes). Moreover, the platform required a very low sample volume for the readout.
Study significance
The study describes the development and validation of a versatile, cost-effective electrochemical sensor platform that combines CRISPR-based viral RNA detection and serological assay-based host antibody detection. The diagnostic performance of the platform is equivalent to conventional laboratory-based methods.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Puig H. 2021. Simultaneous detection of SARS-CoV-2 RNA and host antibodies enabled by a multiplexed electrochemical sensor platform, medRxiv, https://doi.org/10.1101/2021.09.01.21262387, https://www.medrxiv.org/content/10.1101/2021.09.01.21262387v1
- Peer reviewed and published scientific report.
Najjar, Devora, Joshua Rainbow, Sanjay Sharma Timilsina, Pawan Jolly, Helena de Puig, Mohamed Yafia, Nolan Durr, et al. 2022. “A Lab-On-a-Chip for the Concurrent Electrochemical Detection of SARS-CoV-2 RNA and Anti-SARS-CoV-2 Antibodies in Saliva and Plasma.” Nature Biomedical Engineering 6 (8): 968–78. https://doi.org/10.1038/s41551-022-00919-w. https://www.nature.com/articles/s41551-022-00919-w.