Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, it has been noted that advanced age increases the risk of severe complications associated with COVID-19. In advanced age, immune cells undergo changes that result in a vulnerability to infectious diseases, severe disease progression, and poor clinical outcomes. This process is termed immunosenescence and is associated with reduced numbers of naïve B- and T-cells, cytokine dysregulation, defective responses by innate immune subsets, and chronic inflammation due to the accumulation of senescent cells.
To explore the effects of aging on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, several studies involving non-human primates have been conducted. One study on rhesus macaques explains that transcriptional profiling of their lungs 14 days after initial infection found that genes associated with Notch signaling and type-I-interferon were more upregulated in younger animals as compared to older animals, thereby suggesting that age plays a role in regulating these pathways.
In a recent study published on the preprint server bioRxiv*, the authors explore the relationship between host age and immune response to COVID-19 by conducting a time-resolved evaluation of disease in age-stratified rhesus macaques. The authors attempted to build on previous research by obtaining and testing local and systemic tissue, blood, and bronchoalveolar lavage fluid (BALF) samples.
Study methodology
To effectively evaluate the effect age has on the severity of COVID-19, the authors selected eight aged and eight subadult rhesus macaques to be infected with SARS-CoV-2. A virus dilution of 4x105 TCID50/mL in sterile Dulbecco’s Modified Eagle Medium (DMEM) was administered to all animals through a combination of the ocular, oral, intranasal, and intratracheal routes.
Daily observations were taken of the animals and their clinical signs were scored using a standardized scoring sheet. The animals were also clinically examined on days 0, 1, 3, 5, 7, 10, 14, 17, and 21 post-infection. The animal’s temperature, respiration rate, and body weight were the clinical parameters implemented for the examination days.
Study findings
According to the standardized clinical scoring, older animals displayed increased scores when compared to younger animals. From the clinical examinations, it was observed that by day 21 post-infection, all older animals were still showing signs of mild COVID-19, whereas 3 out of 4 younger animals appeared to have fully recovered. Radiographs also showed increased pulmonary infiltrations in the older animals.
To assess the status of viral replication in the respiratory tracts of the animals, samples were taken and the qualitative reverse transcriptase-polymerase chain reaction (qRT-PCR) assay was performed. In the younger animals, the viral genomic ribonucleic acid (gRNA) clearance from the BALF in the lower respiratory tract was consistent. However, in the cohort of older animals, significant variability was observed.
The authors performed single-cell RNA sequencing in BALF to identify the immune cells present in the lower respiratory tract because of the infection. All major immune cells were detected, including B- and T-cells, macrophages, and neutrophils.
On day 3 post-infection, a substantial difference in the frequency of epithelial cell populations was noted in the older animals, whereas in the younger animals, a higher frequency of BALF T-cells was detected. Also on day 7, the older animals displayed heightened levels of all immune cells, especially monocytes and macrophages, which would suggest inflammation. The immune cells of the younger animals had all returned to baseline levels by day 7, which indicates better regulation of inflammatory responses.
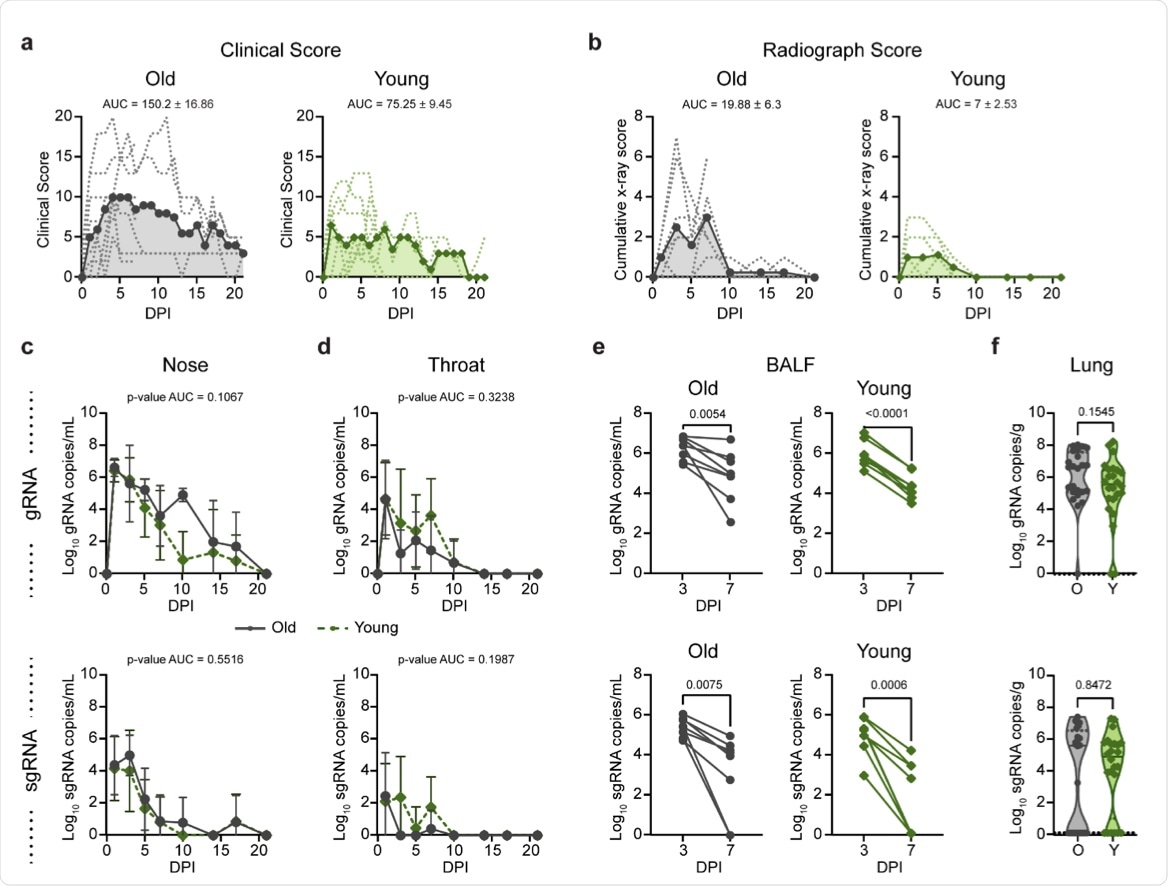 (a) Comparison of clinical scores (y-axis) over the time course of infection (x-axis). Data from individual animals are indicated by dashed lines, group means are shown using solid lines, and the AUC is represented by shading. (b) Ventro-dorsal and lateral radiographs were taken on clinical exam days and scored for the presence of pulmonary infiltrates by a clinical veterinarian according to a standardized scoring system. Individual lobes were scored and scores per animal per day were totaled and displayed in the same format as panel a. (c) Genomic RNA (gRNA, top) and subgenomic RNA (sgRNA, bottom) detected in nasal swabs after inoculation. Data points indicate the geometric mean; error bars represent standard deviation. (d) gRNA and sgRNA quantification in throat swabs. (e) gRNA and sgRNA quantification in bronchoalveolar lavage fluid (BALF). Matched values for individual animals at 3 and 7 dpi are indicated by a connecting line. (f) gRNA and sgRNA quantification in lung samples collected at 7 days post inoculation (dpi). Points represent a single lung lobe section in an individual animal. Grey represents older (O) and green represents younger (Y) rhesus macaques. P-values were calculated using an unpaired t-test test comparing the AUC values (c, d), paired t-test (e), or Mann-Whitney test (f).
(a) Comparison of clinical scores (y-axis) over the time course of infection (x-axis). Data from individual animals are indicated by dashed lines, group means are shown using solid lines, and the AUC is represented by shading. (b) Ventro-dorsal and lateral radiographs were taken on clinical exam days and scored for the presence of pulmonary infiltrates by a clinical veterinarian according to a standardized scoring system. Individual lobes were scored and scores per animal per day were totaled and displayed in the same format as panel a. (c) Genomic RNA (gRNA, top) and subgenomic RNA (sgRNA, bottom) detected in nasal swabs after inoculation. Data points indicate the geometric mean; error bars represent standard deviation. (d) gRNA and sgRNA quantification in throat swabs. (e) gRNA and sgRNA quantification in bronchoalveolar lavage fluid (BALF). Matched values for individual animals at 3 and 7 dpi are indicated by a connecting line. (f) gRNA and sgRNA quantification in lung samples collected at 7 days post inoculation (dpi). Points represent a single lung lobe section in an individual animal. Grey represents older (O) and green represents younger (Y) rhesus macaques. P-values were calculated using an unpaired t-test test comparing the AUC values (c, d), paired t-test (e), or Mann-Whitney test (f).
Analysis of the transcriptional changes occurring in the dividing cells in BALF revealed the older animals had increased numbers of dividing macrophages, while the younger animals showed more dividing CD8+ T-cells. This shows that the younger animals were capable of recruiting T-cells more efficiently than the older animals.
On day 21 post-infection, the authors performed multiplex immunohistochemistry on lung tissue samples from the animals. The younger animals displayed a higher frequency in the expansion of bronchus-associated lymphoid tissue (BALT) when compared to the older animals. Although BALT was also found in the older animals, it was much less abundant, smaller in size, and lacked proliferative activity.
Implications
Although the mechanism of age-related risk to COVID-19 is not completely understood, the findings from this study highlight that age is a definite risk factor. It could be suggested from these results that dysregulated and/or impaired immune responses in the elderly are major contributing factors to the increased risk of COVID-19.
It is evident that there is a direct correlation between the characteristics of immunosenescence displayed at baseline with the older animals and the immune response dynamics, thereby indicating a decline in immune system efficiency and the control of homeostasis. In the older animals, the dampened immune responses may also be a consequence of the limited priming capacity seen in advanced age due to depleted numbers of naïve T-cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Speranza, E., Purushotham, J. N., Port, J. R., et al. (2021). Age-related differences in immune dynamics during SARS-CoV-2 infection in rhesus macaques. bioRxiv. doi:10.1101/2021.09.08.459430. https://www.biorxiv.org/content/10.1101/2021.09.08.459430v1
- Peer reviewed and published scientific report.
Speranza, Emily, Jyothi N. Purushotham, Julia R. Port, Benjamin Schwarz, Meaghan Flagg, Brandi N. Williamson, Friederike Feldmann, et al. 2022. “Age-Related Differences in Immune Dynamics during SARS-CoV-2 Infection in Rhesus Macaques.” Life Science Alliance 5 (4). https://doi.org/10.26508/lsa.202101314. https://www.life-science-alliance.org/content/5/4/e202101314.