Researchers in the UK have conducted a large population-based study showing that people with immune-mediated inflammatory diseases (IMIDs) appear to be at an increased risk of serious outcomes from coronavirus disease 2019 (COVID-19).
The study of more than 17 million people found that between March and September 2020, individuals with IMIDs were at an increased risk of COVID-19-related death, critical care admission and hospitalization, compared with the general population.
The researchers did not find any increased risk of adverse COVID-19 outcomes among those taking most targeted immune-modifying drugs, compared with standard systemic treatments.
However, the use of rituximab was associated with an increased risk of COVID-19 death and critical care admission.
“Our study offers insights into future risk mitigation strategies and SARS-CoV-2 vaccination priorities for individuals with IMIDs, as it highlights that those with IMIDs and those taking rituximab may be at risk of severe COVID-19 outcomes,” writes the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
More about severe COVID-19 disease risk among those with IMIDs
Although the majority of people with COVID-19 experience mild symptoms, approximately 15% develop pneumonia requiring hospitalization and around 5% progress to severe disease.
It is not yet clear whether people with IMIDs such as inflammatory joint, bowel and skin diseases or taking immune-modifying treatments are at an increased risk of severe outcomes.
Early in the pandemic, the UK government issued guidance regarding “shielding” to limit the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes COVID-19.
Individuals considered at high risk of severe disease outcomes were advised to stay at home to minimize their face-to-face contact with others.
Shielding advice was directed at people identified (using health records) as having specific immunosuppressive diseases or taking prescribed immune-modifying drugs.
Disease-specific registries have been used to assess COVID-19 risks in these individuals, and results have so far been reassuring.
“However, disease-specific registries are limited by small sample sizes, inherent selection bias, and lack of denominators,” says Langan and colleagues.
The OpenSAFELY-TPP platform is a new analytics platform for electronic health records that is used to deliver pandemic-related research.
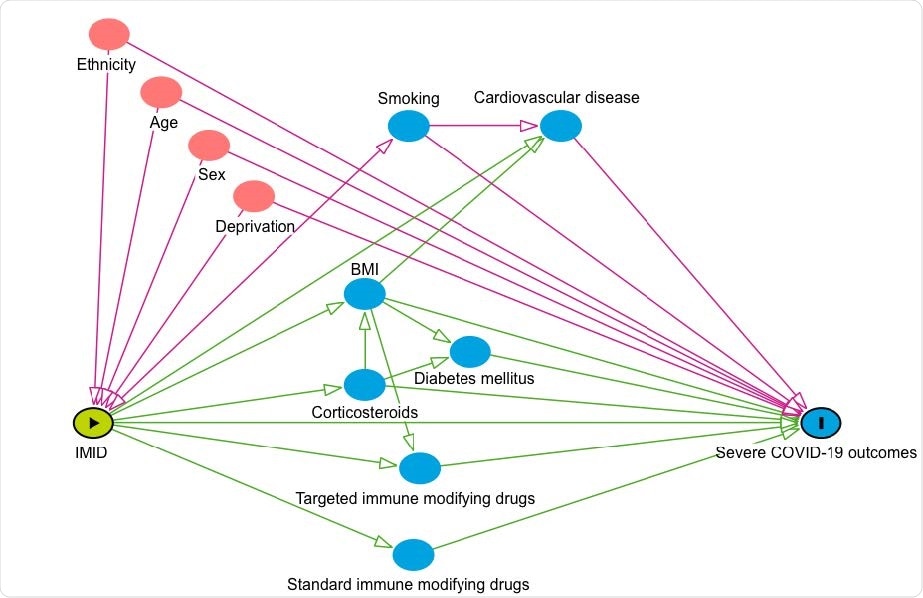
Conceptual framework: risk of COVID-19-related death in people with IMIDs compared to the general population. The conceptual framework represents the assumed associations between covariates and primary exposure and outcome. Pink circles represent ancestors of the exposure and outcome, blue circles represent ancestors of the outcome, pink lines represent biasing paths (i.e. confounding) and green lines represent causal paths. The minimally sufficient adjustment set (i.e. the covariates adjusted for in confounder adjusted models) represents covariates such that the adjustment for this set of variables will minimize confounding bias when estimating the association between the exposure and the outcome.
What did the current study involve?
Langan and colleagues used OpenSAFELY to analyze primary care data linked to hospital admission, death and prescription data to compare the risk of COVID-19-related death, critical care admission and hospitalization among people with IMIDs and the general population between March 1st and September 30th, 2020.
The study identified 17,672,065 adults (aged 18 to 110 years), 1,163,438 (7%) of whom had IMIDs. Of those with IMIDs, 17% had inflammatory joint diseases (rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis), 17% had inflammatory bowel diseases (Crohn’s, ulcerative colitis, or unclassified IBD) and 66% had inflammatory skin disease (psoriasis or hidradenitis suppurativa).
Of these individuals, 19,119 received targeted immune-modifying drugs, and 200,813 received standard systemic therapies.
People with IMIDs were at an increased risk of severe COVID outcomes
After adjusting for age, sex, deprivation and smoking status, the researchers found that people with IMIDs were at a 23% greater risk for COVID-19-related death, a 24% greater risk of critical care admission or death and a 32% increased risk of hospitalization, compared with the general population.
The increased risk of death was greatest for inflammatory joint disease (47% increase), with smaller effect estimates observed for inflammatory skin disease (12%) and bowel disease (12%.)
The increased risk of critical care admission or death was also greatest for those with inflammatory joint disease (46% increase), with smaller effect estimates observed for skin (15%) and bowel disease (13%).
For COVID-19 hospitalization, the risk increase was 53% for inflammatory joint disorders, 21% for skin disease and 3% for bowel disease.
Targeted versus systemic immune-modifying treatments
Compared with people taking standard systemic treatments, there was no evidence of an increased risk for severe COVID-19-related outcomes among those taking tumor necrosis factor (TNF) inhibitors, interleukin (IL)-12/23, IL7, IL-6 or Janus kinase (JAK) inhibitors.
However, rituximab was associated with an increased risk of COVID-19-related death and hospital admissions, although the authors say this this finding may be related to worse underlying health rather than the drugs themselves.
“The increased risk of adverse COVID-19 outcomes that we saw in people with IMIDs and those treated with rituximab merits further study,” says Langan and colleagues.
What did the team conclude?
The researchers say the findings provide an evidence base to inform policy on booster vaccination prioritization and risk-mitigating behavior advice, but must be interpreted in the context of UK public health policy on shielding.
“The findings will support healthcare professionals engaging in shared decision making and communication of risk,” they conclude.
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Langan S, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune modifying therapies: a nationwide cohort study in the OpenSAFELY platform. medRxiv, 2021. doi: https://doi.org/10.1101/2021.09.03.21262888, https://www.medrxiv.org/content/10.1101/2021.09.03.21262888v1
- Peer reviewed and published scientific report.
MacKenna, Brian, Nicholas A Kennedy, Amir Mehrkar, Anna Rowan, James Galloway, Julian Matthewman, Kathryn E Mansfield, et al. 2022. “Risk of Severe COVID-19 Outcomes Associated with Immune-Mediated Inflammatory Diseases and Immune-Modifying Therapies: A Nationwide Cohort Study in the OpenSAFELY Platform.” The Lancet Rheumatology 4 (7): e490–506. https://doi.org/10.1016/s2665-9913(22)00098-4. https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(22)00098-4/fulltext.