The coronavirus disease 2019 (COVID-19) has been especially catastrophic for the elderly, who are often considered to be at the greatest risk of severe disease. One of the most significant ways in which the current pandemic can be brought to an end has been through the development and production of effective vaccines.
The BNT162b2 vaccine produced by Pfizer, as well as the mRNA-1273 vaccine produced by Moderna, both operate under the same principle. To this end, these are both messenger ribonucleic acid (mRNA) vaccines that produce immunity by providing the cell with the mRNA of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein.
The cellular machinery then translates the mRNA, thus leading to the production of the protein. This ultimately allows the immune system to develop a robust immune response against SARS-CoV-2. As no viral structural proteins are involved in this process, and as mRNA is non-infectious and non-integrating, this approach is considered to be safer than traditional vaccines created from inactive strains of the virus, which can reactivate or cause allergic reactions.
About the study
Following observations in alternate vaccines showing poor immunity in older individuals, researchers from Simon Fraser University in Canada decided to investigate the magnitude of this effect in mRNA vaccines for COVID-19. In total, these researchers examined the responses from 151 individuals, including healthcare workers, older adults, and those living in long-term care and assisted living facilities.
Serum and plasma were collected from these individuals prior to vaccination, one month after the first dose, and three months after the second dose. Those who were infected by SARS-CoV-2 when the study began were identified by the presence of antibodies recognizing the SARS-CoV-2 nucleoprotein.
A preprint version of this study is currently available on the medRxiv* server.
To examine the immune response, the scientists performed binding antibody assays against the SARS-CoV-2 nucleoprotein and the spike protein receptor-binding domain (RBD), as well as quantified the immunoglobulin G (IgG) response using bead-based enzyme-linked immunosorbent assays (ELISAs).
SARS-CoV-2 enters human cells through the binding of its spike protein RBD to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell. Thus, this interaction is an important aspect of vaccine-mediated immunity, as vaccine efficacy is often measured by how well plasma antibodies can block this interaction.
The binding between the SARS-CoV-2 RBD and ACE2 receptors was measured through competition ELISAs, where bound ACE2 was detected using streptavidin-PE. The activity of neutralizing antibodies was detected through assays to detect the viral cytopathic effect, which describes viral-induced death of the cell through lysis or inability to reproduce.
Study findings
The researchers found that following the first dose, median anti-RBD IgG titers were 4.2 fold lower in older adults compared to healthcare workers. The exception to this was the 14 individuals who had been infected with SARS-CoV-2 prior to being vaccinated, who showed between 190- and 840-fold higher IgG responses, a finding that is supported by previous studies showing intense immune response to COVID-19 in individuals who had been previously infected and received one dose of the COVID-19 vaccine.
After two doses, the antibody titers of the naïve healthcare workers reached the same reactivity as the convalescent individuals. Unfortunately, older individuals still showed 3-fold lower IgG activity compared to healthcare workers, although the boost in immunity provided by the second dose did appear to be greater for the elderly.
When examining the total anti-spike protein RBD antibodies, very similar results were seen between all groups. To this end, a significantly lower binding antibody response was seen in older adults after both one and two doses.
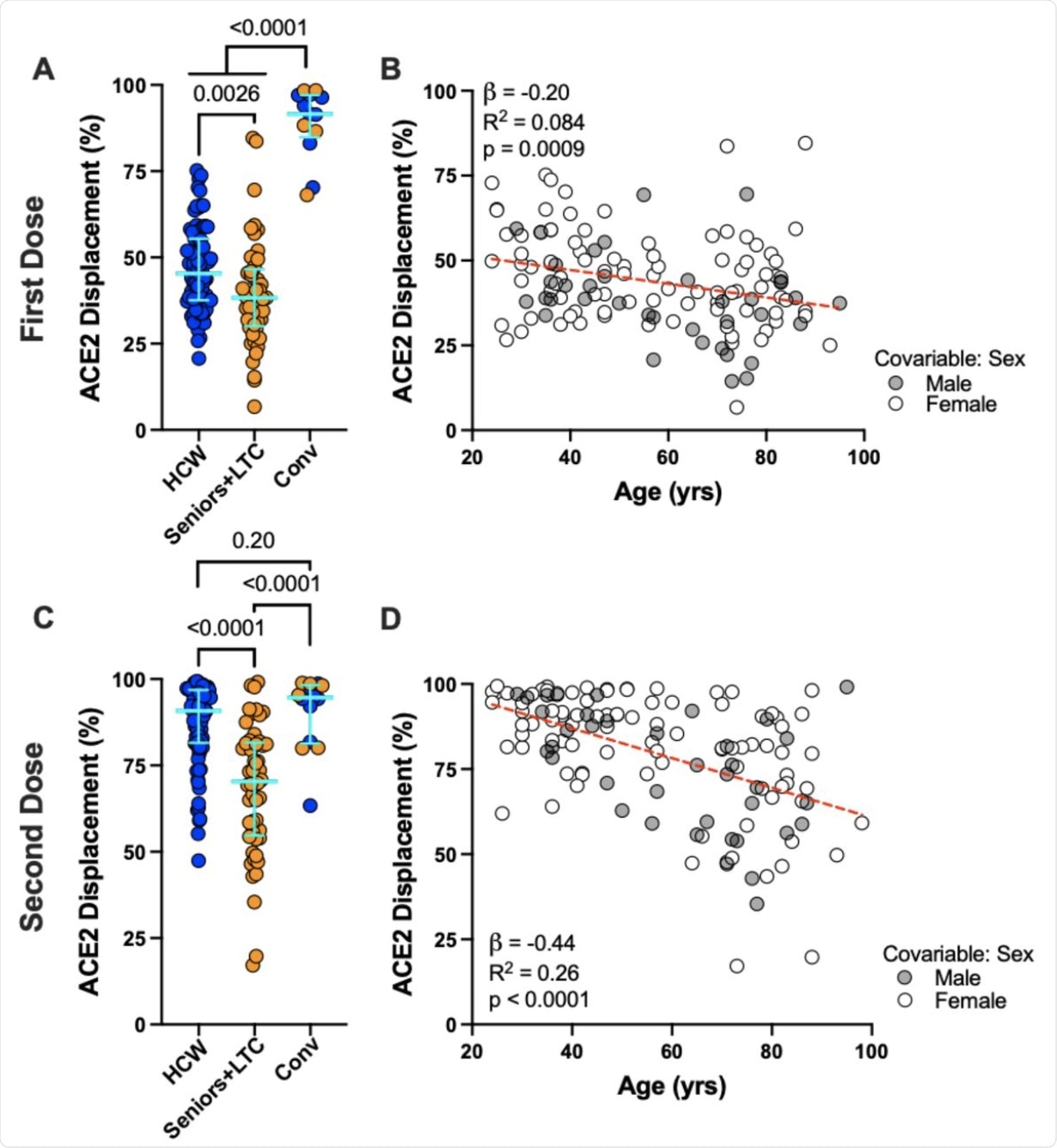 Panel A: Ability of vaccine-induced plasma antibodies to displace soluble ACE2-receptor from spike RBD, measured using a Luminex ELISA assay, following one dose of vaccine in COVID-19 naive Seniors+LTC (orange circles), HCW (blue circles), and COVID-19 convalescent participants (“Conv”; colored as above). Bars represent median and IQR. P-values computed using the Mann-Whitney U-test. Panel B: Same data as the HCW and Seniors+LTC groups shown in panel A, but plotted by age, and colored by sex, which remained significant in multivariable analyses (see Table 2). Statistics computed using ordinary least-squares regression, also shown as dotted line. Panels C, D: Same as A and B, but for responses following two doses of mRNA vaccine.
Panel A: Ability of vaccine-induced plasma antibodies to displace soluble ACE2-receptor from spike RBD, measured using a Luminex ELISA assay, following one dose of vaccine in COVID-19 naive Seniors+LTC (orange circles), HCW (blue circles), and COVID-19 convalescent participants (“Conv”; colored as above). Bars represent median and IQR. P-values computed using the Mann-Whitney U-test. Panel B: Same data as the HCW and Seniors+LTC groups shown in panel A, but plotted by age, and colored by sex, which remained significant in multivariable analyses (see Table 2). Statistics computed using ordinary least-squares regression, also shown as dotted line. Panels C, D: Same as A and B, but for responses following two doses of mRNA vaccine.
A slightly lower effect size was seen in the ability of plasma to block the RBD-ACE2 interaction. Previously infected individuals showed 92% ACE2 displacement activity following one dose, while older adults and healthcare workers were at 38% and 45%, respectively.
After two doses, the difference became more pronounced, with healthcare workers showing 91% displacement activity and seniors exhibiting this same activity at 70%. Interestingly, the levels of binding antibodies seen in seniors were equal to those in healthcare workers; however, the antibodies in seniors had a far lesser ability to displace ACE2. On average, every 10-year increase in age was associated with a 2.0% and 4.4% lower displacement activity following one and two vaccines doses, respectively.
A more striking difference could be seen in the neutralizing activity of plasma. Following one dose, 21% of healthcare workers showed evidence of neutralizing activity, which was comparable to only 3% of seniors. This trend continued following the second dose, with all healthcare worker plasma showing the ability to prevent virus infection of target cells, while only 65% of plasma gathered from seniors showing the same ability.
Both older adults and healthcare workers showed reduced immunity against the Delta variant as compared to the Wuhan strain. This observation has been supported by previous studies.
Conclusion
The authors emphasize the importance of their study in planning both future vaccine design, as well as vaccine deployment. They also emphasize the ongoing infection risk for older adults, as even after two doses failed to completely neutralize SARS-CoV-2. This emphasizes the importance of both booster doses and continued efforts to halt the spread of SARS-CoV-2.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Brockman A., Mwimanzi, F., Lapointe, H. R., et al. (2021) A reduced magnitude and durability of humoral immune responses by COVID-19 mRNA vaccines among older adults. doi:10.1101/2021.09.06.21263149. https://www.medrxiv.org/content/10.1101/2021.09.06.21263149v1.
- Peer reviewed and published scientific report.
Brockman, Mark A, Francis Mwimanzi, Hope R Lapointe, Yurou Sang, Olga Agafitei, Peter K Cheung, Siobhan Ennis, et al. 2021. “Reduced Magnitude and Durability of Humoral Immune Responses to COVID-19 MRNA Vaccines among Older Adults.” The Journal of Infectious Diseases 225 (7): 1129–40. https://doi.org/10.1093/infdis/jiab592. https://academic.oup.com/jid/article/225/7/1129/6458430.