Recent studies indicate that individuals with obesity, especially those having predominant visceral adipose tissue (VAT) accumulation, are at a higher risk of developing more severe cases of the coronavirus disease 19 (COVID-19).
 Study: Antibody responses to BNT162b2 mRNA vaccine: infection-naïve individuals with abdominal obesity warrant attention. Image Credit: MattLphotography / Shutterstock.com
Study: Antibody responses to BNT162b2 mRNA vaccine: infection-naïve individuals with abdominal obesity warrant attention. Image Credit: MattLphotography / Shutterstock.com
Background
The messenger RNA (mRNA) vaccines that are currently being administered for protection against COVID-19 have been found to be quite effective in preventing the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, as well as promoting the development of herd immunity. However, there has been fear among researchers on the effectiveness of these vaccines in people with obesity, as studies on hepatitis B, influenza, and rabies have shown that the immune response is reduced in these individuals.
Although no study to date has shown altered efficacy of the mRNA vaccines in individuals with obesity as compared to those without obesity, most of these studies were based on an assessment of body mass index (BMI). BMI is not considered the best indicator of adiposity, as the distribution and amount of body fat are not taken into account.
A new study published on the preprint server medRxiv* evaluated the antibody response against SARS-CoV-2 after administration of the first and second dose of the BNT162b2 mRNA vaccine in healthcare workers. Comparison of the response was also conducted between individuals who were affected by abdominal obesity (AO) and those who were not, irrespective of whether or not these individuals had a prior SARS-CoV-2 infection.
About the study
The current study involved the collection of blood samples from healthcare workers over the age of 18 who had received two doses of the Pfizer-BioNTech BNT162b2 vaccine. Pregnancy was an important exclusion factor of the study. Additionally, data on medical history, smoking status, and pharmacotherapy was collected from all participants.
The waist circumference of the participants was measured midway between the lower rib and the iliac crest closest to 1 cm. For the determination of AO, the cut-off was set at 94 cm in men and 80 cm in women. BMI was calculated by dividing weight by height square.
The measurement of the level of antibodies took place at four different time points: at baseline, day 21 of receiving the first dose of the vaccine, and at one month and three months of receiving the second dose of the vaccine.
Furthermore, serological testing was done to determine the presence of immunoglobulin G (IgG) neutralizing antibodies against the Trimeric complex (IgG-TrimericS), which includes the N-terminal and receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein. Nucleocapsid antibodies were used for the detection of any prior infection.
Study findings
The results of the study indicate that, among infection-naïve individuals at baseline, no difference in the level of IgG-TrimericS was observed between individuals with and without AO. However, at other times, IgG-TrimericS levels were lower in individuals with AO as compared to individuals without AO.
Two noteworthy observations were made in infection-naïve individuals. The first observation was that after one month of receiving their second dose of the mRNA vaccine, individuals with AO showed a lower peak of IgG-TrimericS levels as compared to individuals without AO. The second observation was that between the first and third month of receiving the second dose of the vaccine, a greater drop in IgG-TrimericS levels was observed in individuals with AO as compared to individuals without AO.
The results involving individuals with prior infections were quite different. It was observed that individuals with AO showed higher levels of IgG-TrimericS than individuals without AO at baseline.
The results also indicated that among individuals with AO, IgG-TrimericS levels in individuals with prior infection at baseline were slightly lower than in infection-naïve individuals after receiving the first dose of the vaccine. Also, the levels of IgG-TrimericS in infection-naïve individuals who had received the second dose of the vaccine were significantly lower than previously infected individuals who had received the first dose of the vaccine.
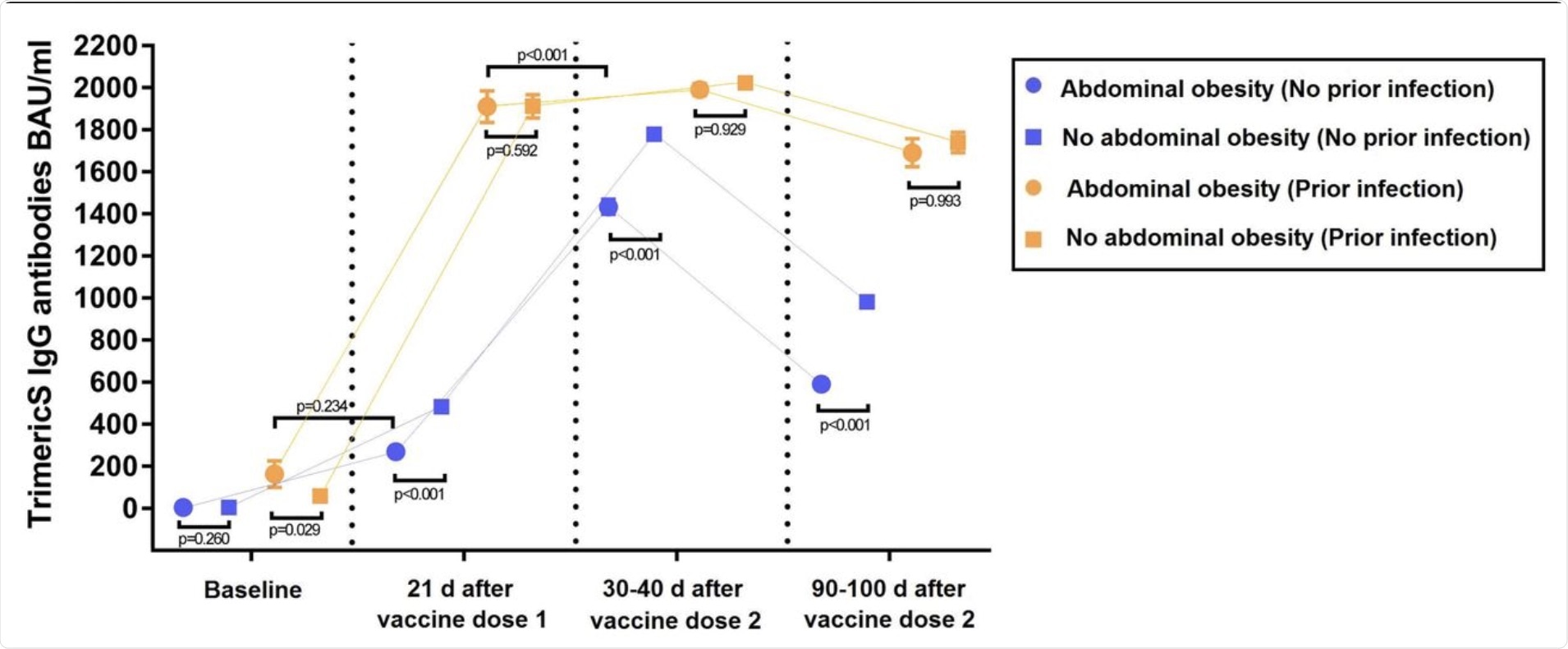 IgG-TrimericS antibody response to mRNA SARS-CoV-2 vaccination in individuals with or without abdominal obesity, discerning between individuals with or without prior SARS-CoV-2 infection.
IgG-TrimericS antibody response to mRNA SARS-CoV-2 vaccination in individuals with or without abdominal obesity, discerning between individuals with or without prior SARS-CoV-2 infection.
The study had certain limitations; firstly, measurement of virus-specific T-cells was not conducted. Secondly, the assessment of anti-N antibodies was only conducted once before SARS-CoV-2 infection. Thirdly, the evaluation of pro-inflammatory markers was not conducted.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.