Despite the advent of numerous vaccines and the discovery of passive monoclonal antibodies for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), there remains an urgent need for definitive therapies for coronavirus disease-2019 (COVID-19) patients.
Present global challenges in combatting the pandemic include – the emergence of viral variants; declining immunity following vaccination; vulnerability of immunocompromised individuals to severe symptoms even after vaccination; and chronic or persistent infection that generate variants that can escape immunity.
Anti-inflammatory drugs exhibit only slight clinical benefit in certain subgroups of patients. Therefore, the control and eradication of this viral spread is the need of the hour.
Mupadolimab is an IgG1κ humanized FcγR binding-deficient anti-CD73 monoclonal antibody (mAb) that activates CD73POS B cells. Mupadolimab is known to increase the expression of markers associated with B cell maturation and antigen presentation and increase the secretion of Immunoglobulin M (IgM) and Immunoglobulin G (IgG), in vitro.
Researchers across various institutions in the U.S undertook a study and to examine the use of mupadolimab for boosting anti-viral immune responses and improving clinical outcomes in COVID-19. The study is posted online on the medRxiv* server while awaiting peer review.
ddd
This was an open-label phase 1 trial with mupadolimab in hospitalized COVID-19 patients, the findings of which led to the initiation of a randomized double-blinded, placebo-controlled phase 3 study. However, the latter was terminated early in mid-2021 due to the declining number of COVID-19 cases in the U.S.
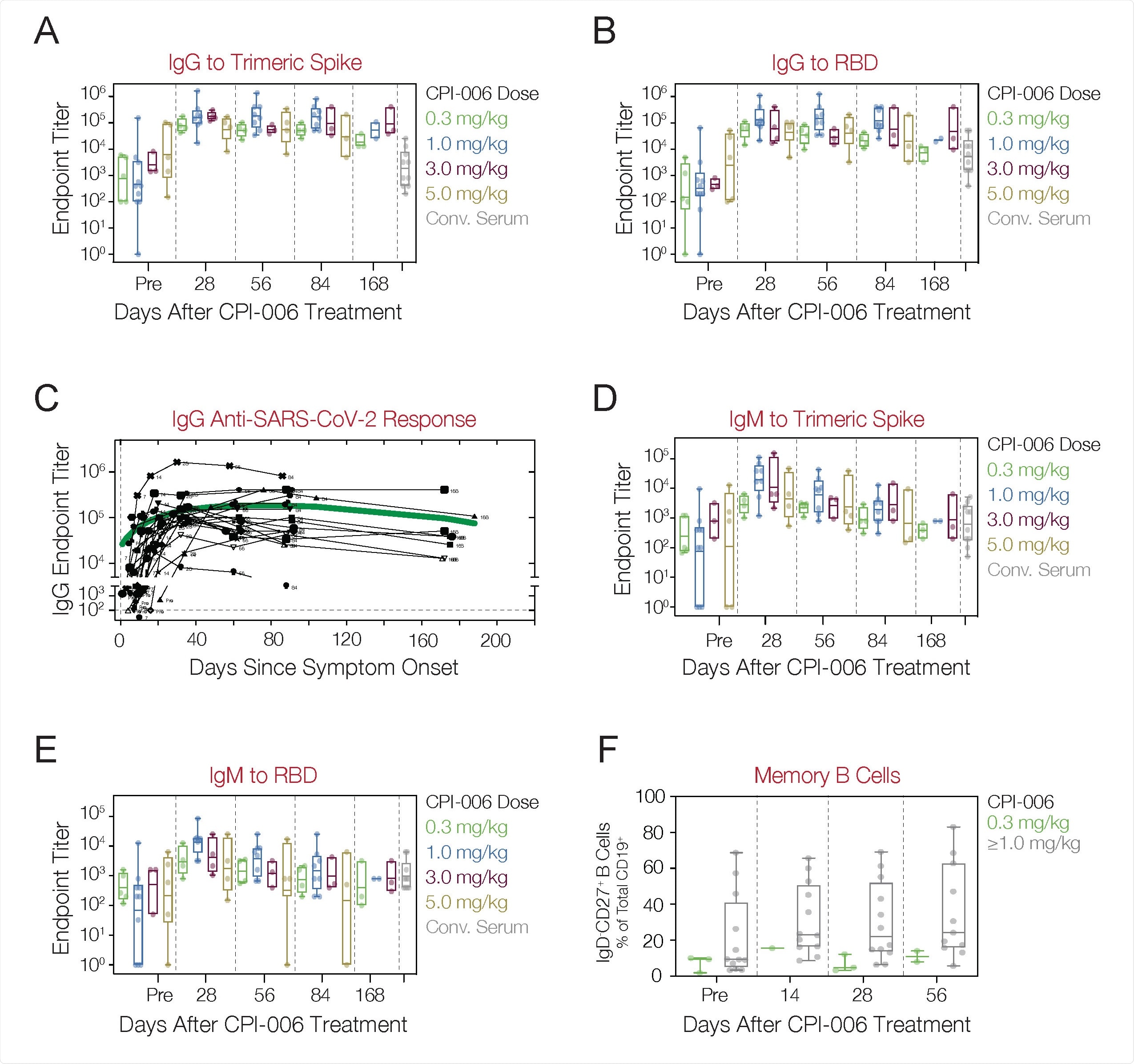
Anti-SARS-CoV-2 antibody and cellular responses in COVID-19 patients treated with mupadolimab. A, B) Patients received a 0.3, 1.0, 3.0 or 5.0 mg/kg single dose of mupadolimab and endpoint IgG titers to TS (A) and RBD (B) were measured at pre-treatment and at day 28, 56, 84, and 168. C) Longitudinal analyses of IgG against SARS-CoV-2 in mupadolimab treated subject. The green line is a smoothing spline fit. Each black line represents an individual patient. D, E) Patients received a 0.3, 1.0, 3.0 or 5.0 mg/kg single dose of mupadolimab and endpoint IgM titers to TS (D) and RBD (E) were measured at pre-treatment and at day 28, 56, 84, and 168. F) Frequency of circulating memory B cells (CD19POSIgDNEGCD27POS) within CD19POS gate at baseline and after treatment in patients treated with 0.3 mg/kg mupadolimab compared to ≥1.0 mg/kg. Data are shown as box and whisker plot with geometric mean and interquartile range. Each dot represents a patient. Also shown are titers from convalescent patient serum obtained 4- 6 weeks after POS.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Here, an animal model was utilized to determine whether mupadolimab could enhance antigen-specific immune responses to SARS-CoV-2. It was observed that mice vaccinated with the trimeric spike protein (TS) plus mupadolimab made antigen-specific human anti-TS antibodies. In contrast, those receiving TS plus isotype control did not elicit a response. These antibody responses were antigen-specific.
The results indicated that mupadolimab induced antigen-specific humoral immunity—to immunizing TS protein—and has the potential to trigger antibody responses to SARS-CoV-2 in patients with COVID-19.
Immunotherapy of COVID-19 – Phase 1 open-label trial
The Phase 1 open-label, single dose-escalation trial evaluated the safety, immunologic effects, and clinical outcomes in patients hospitalized with mild-to-moderate COVID-19. Cohorts of patients were administered intravenous infusions of 0.3 mg/kg, 1.0 mg/kg, 2.0 mg/kg, 3.0 mg/kg or 5.0 mg/kg of mupadolimab, given over 5-10 minutes.
It was found that the time-range post-onset of symptoms (POS) to mupadolimab administration was 1-21 days, with a median of 8 days. No drug-related adverse events or changes in quantitative serum immunoglobulins were reported. All patients recovered with an improvement in inflammatory markers and symptoms and were discharged at a median of 3 days. No patients required invasive or non-invasive mechanical ventilation.
Immune Responses in Mupadolimab Treated COVID-19 Patients
The IgG antibody titers against the SARS-CoV-2 TS and/or receptor-binding domain (RBD) were markedly increased in all patients, 28 days after a single infusion of low doses of mupadolimab. At the same time, lower antibody titers were recorded in the cohort receiving 0.3 mg/kg. Serum concentrations of mupadolimab achieved levels exceeding 1 µg/ml for greater than 24 hours for doses of 1 mg/kg and higher – concentrations known to activate B cells in vitro. Besides, IgG titers sustained without depletion up to six months after symptom onset.
Furthermore, antibody titers were higher with this therapy than with convalescent sera from recovered patients. Anti-SARS-CoV-2 IgM titers showed a similar trend with decreasing titers observed beyond 84 days.
Preliminary evidence suggested that mupadolimab increased the frequency of memory B cells. However, memory phenotype-B cells did not increase in the lowest dose group.
Prolonged and elevated neutralizing titers were observed in patients following mupadolimab treatment – with ID50 values up to 24 000 that persisted more than 56 days after symptom onset. In addition, day-28 serum from mupadolimab treated patients effectively neutralized the B.1.1.7 variant.
The findings suggested that mupadolimab treatment can elicit a robust and durable polyclonal neutralizing antibody response in COVID-19 patients, which can render cross-protection against emerging SARS-CoV-2 variants.
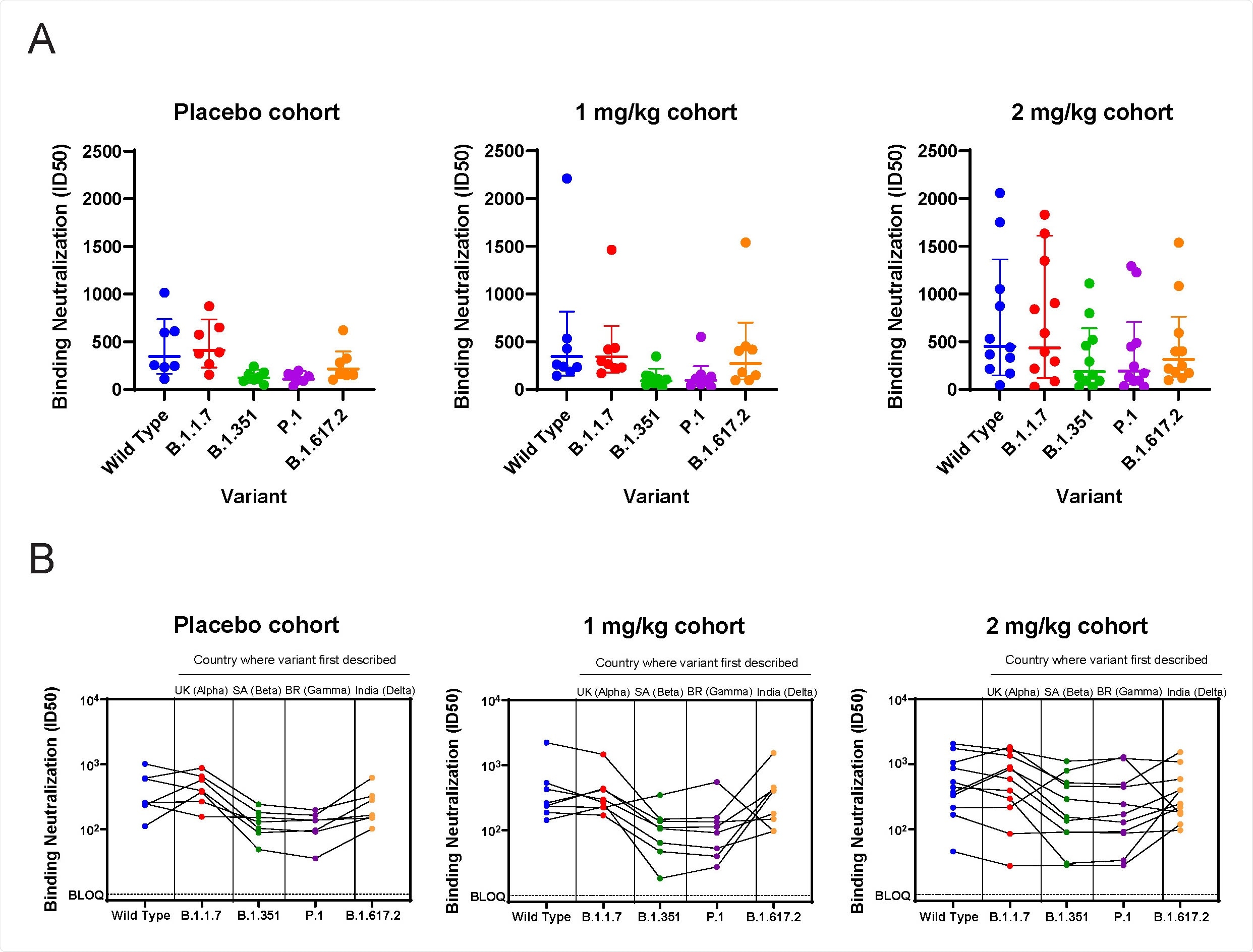
ACE2 / RBD blocking assay. A) ID50 for serum samples assayed against wild type (Wuhan) and variants using ACE2-RBD blocking assay. B) As in A, with data showing reactivity for each patient against variants.
Randomized Double-Blind Placebo-Controlled Trial
A multicenter, stratified study of mupadolimab plus standard of care (SOC) versus placebo plus SOC, in mild to moderately symptomatic hospitalized patients with COVID-19 was undertaken. Here, the three treatment arms received intravenous mupadolimab – 2 mg/kg, 1 mg/kg or placebo delivered by 5-10-minute infusion.
There were no drug-related adverse effects in patients receiving mupadolimab. All endpoints trended toward a more favorable outcome for mupadolimab; 93.3%, 85.7%, and 81.1% of patients were alive and free from respiratory failure in the 2 mg/kg, 1 mg/kg mupadolimab, and placebo cohorts, respectively. Mupadolimab also fared better in time to clinical improvement, time to sustained improvement, and time to hospital discharge.
Furthermore, patients given 2 mg/kg of mupadolimab also demonstrated higher cross-reactivity with the B.1.351 and P.1 variants compared to those who received 1 mg/kg mupadolimab and placebo.
Mupadolimab causes the prolonged retention of activated B cells in lymphoid organs and the thymus. Additionally, this drug induces the secretion of cytokines involved in B cell differentiation, including CCL22. It is proposed that mupadolimab restores proper immune function in germinal centers (of impaired humoral immunity) of SARS-CoV-2 infected patients either through its action on B cells or other CD73 positive cells.
The findings depicted an atypically magnanimous and durable antibody response with mupadolimab treatment, which offers a novel approach to treating COVID-19 through the stimulation of B cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Miller R, Guru P, Bauer P et al. Clinical Results with a B Cell Activating Anti-CD73 Antibody for the Immunotherapy of COVID-19. 2021. doi:10.1101/2021.09.13.21263406, https://www.medrxiv.org/content/10.1101/2021.09.13.21263406v1
- Peer reviewed and published scientific report.
Miller, Richard A., Jason John Luke, Shenshen Hu, Suresh Mahabhashyam, William B. Jones, Thomas Marron, Jaime R. Merchan, Brett G. M. Hughes, and Stephen B. Willingham. 2022. “Anti-CD73 Antibody Activates Human B Cells, Enhances Humoral Responses and Induces Redistribution of B Cells in Patients with Cancer.” Journal for ImmunoTherapy of Cancer 10 (12): e005802. https://doi.org/10.1136/jitc-2022-005802. https://jitc.bmj.com/content/10/12/e005802.