The coronavirus disease 2019 (COVID-19) pandemic led to the development of several nucleic acid-based vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The immunogenicity and efficacy of these vaccines may be lower in immunocompromised and sick individuals.
To better understand this phenomenon, a new study published on the preprint server medRxiv* discusses the utility of a third booster dose in patients with myelofibrosis (MF), where the immune response is weaker as compared to healthy individuals.
 Study: The Slower Antibody Response in Myelofibrosis Patients After Two Doses of mRNA SARS-Cov-2 Vaccine Calls for A Third Dose. Image Credit: Corona Borealis Studio / Shutterstock.com
Study: The Slower Antibody Response in Myelofibrosis Patients After Two Doses of mRNA SARS-Cov-2 Vaccine Calls for A Third Dose. Image Credit: Corona Borealis Studio / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The pandemic led to the rapid hospitalization of hundreds of thousands of people with respiratory distress, many of whom progressed to severe inflammation and multi-organ dysfunction. With a mortality rate of 0.5-1%, depending on the country, 4.7 million documented deaths have been caused by COVID-19.
Vaccination has been hailed as one of the only ways that the world can return to some form of normalcy. With unparalleled speed, researchers developed effective vaccines on various platforms, the earliest of which was the Pfizer/BioNTech vaccine. This vaccine gained emergency approval in December 2021, followed closely by the Moderna vaccine.
However, cancer patients, especially those with blood cancers, were highly vulnerable to the virus, with over a third of them dying from COVID-19. For this reason, vaccination has been strongly advised for this group and they have been prioritized for the vaccine.
MF is a stem cell disorder affecting hemopoietic cells in the bone marrow. This condition is found in tumors Philadelphia-negative myeloproliferative neoplasms (MPN) and causes affected patients to be at higher risk of death from COVID-19.
The drug ruxolitinibb, a JAK-STAT signaling pathway inhibitor, has been used in MF to modulate the inflammatory response and reduce cytokine production. Unfortunately, it also weakens the immune response. To this end, ruxolitinib has been explored as a potential treatment option for severe-to-critical COVID-19 to prevent or reduce the cytokine storm, which is often the precursor of fatal acute respiratory distress syndrome (ARDS) and death.
However, the drug cannot be stopped all at once in these patients once they develop COVID-19 because of the rebound spike in cytokines, which can precipitate severe systemic inflammation and death.
Early data failed to arrive at a conclusive result on the immunogenicity of messenger ribonucleic acid (mRNA) vaccines in MF patients. The present study aimed at assessing the antibody response in this patient group against the background of the controversy over giving a third dose to those already vaccinated with two doses of an mRNA vaccine.
Study findings
The current study compared 42 MF vaccine recipients with 40 healthy controls. About 40% of MF vaccine recipients were on ruxolitinib,
The results showed that three out of four MF patients developed immunoglobulin G (IgG) antibodies after two doses of the vaccine. However, the rate of antibody development was slower than that which was detected in healthy individuals, which may indicate that these patients had an impairment of their immune response when stimulated by the vaccine.
At seven days post-vaccination, the geometric mean titer (GMT) of anti-spike IgG antibodies in these patients was low relative to that in healthy controls, irrespective of the use of ruxolitinib, at just over 1,000 versus over 20,000, respectively. Thereafter, it rose until both groups were equal at one month from the second dose, except for those on ruxolitinib.
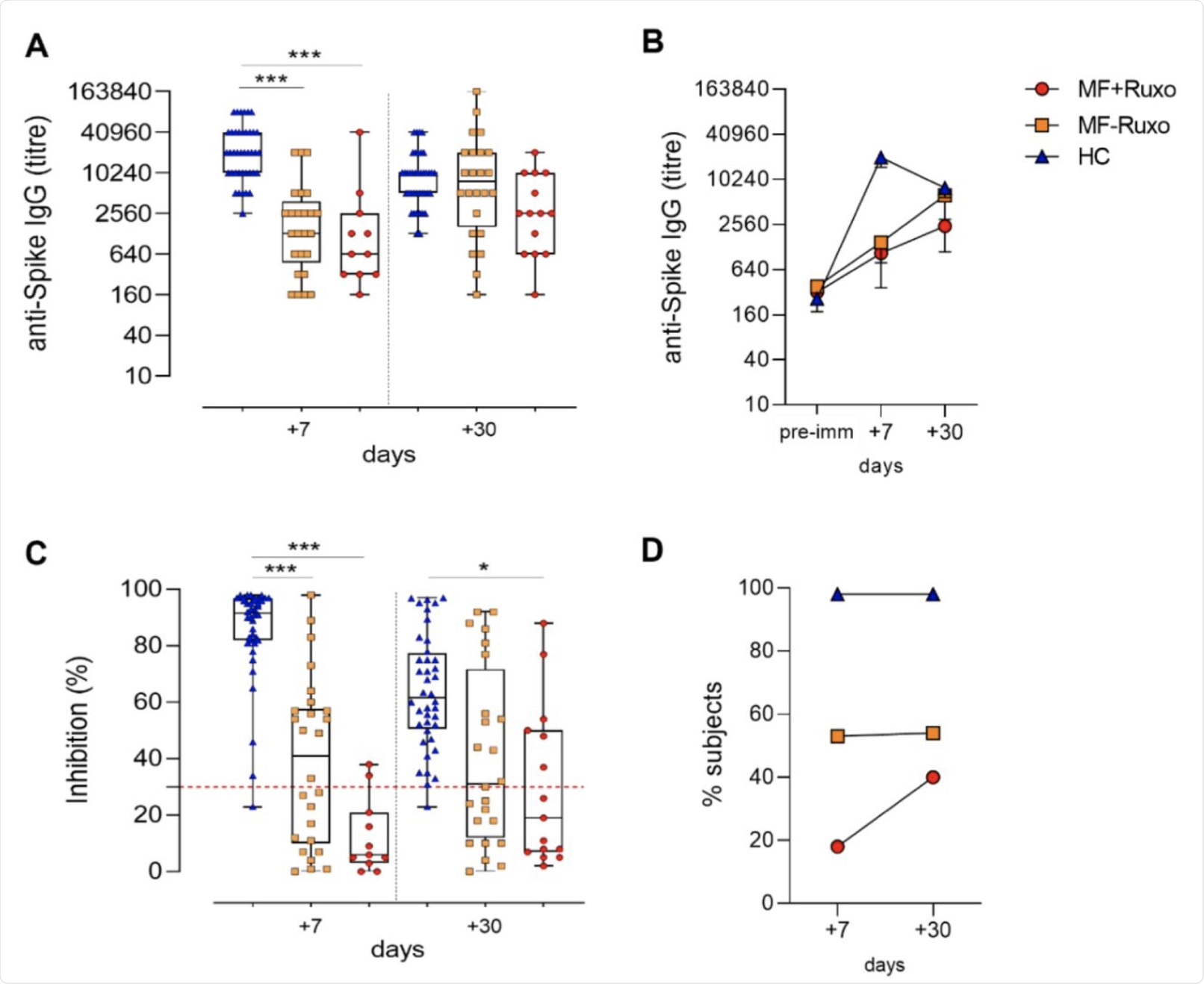 Humoral immune response against SARS-CoV-2 in MF patients post-second dose of mRNA vaccination. A) IgG titers specific for the Spike protein assessed by ELISA in plasma of patients with myelofibrosis (MF) patients, treated or not with ruxolitinib (MF+Ruxo or MF-Ruxo), and Healthy controls (HC), at different time points post the second vaccine dose. Antibody endpoint titers were expressed as the reciprocal of the sample dilution reporting double the background OD value. B) Time course of Spike-specific IgG in plasma collected at the baseline (pre-immune) and 7 and 30 days from the second dose of mRNA SARS-CoV-2 vaccine. C. ACE2/RBD inhibition activity tested in plasma using a SARS-CoV-2 surrogate virus neutralization test. Results are expressed as ACE2/RBD inhibition percentage with box and whiskers diagram showing all subject values. Inhibition values >30% are regarded as positive results according to the manufacturer indication. D. Percentage of subjects developing ACE2/RBD inhibition positive values (>30%) in the different groups. Kruskal-Wallis test, followed by Dunn’s post-test for multiple comparisons, was used for assessing statistical differences between groups. * P≤ 0.05; * * * P ≤ 0.001. Healthy control (HC), myelofibrosis (MF), ruxolitinib (Ruxo).
Humoral immune response against SARS-CoV-2 in MF patients post-second dose of mRNA vaccination. A) IgG titers specific for the Spike protein assessed by ELISA in plasma of patients with myelofibrosis (MF) patients, treated or not with ruxolitinib (MF+Ruxo or MF-Ruxo), and Healthy controls (HC), at different time points post the second vaccine dose. Antibody endpoint titers were expressed as the reciprocal of the sample dilution reporting double the background OD value. B) Time course of Spike-specific IgG in plasma collected at the baseline (pre-immune) and 7 and 30 days from the second dose of mRNA SARS-CoV-2 vaccine. C. ACE2/RBD inhibition activity tested in plasma using a SARS-CoV-2 surrogate virus neutralization test. Results are expressed as ACE2/RBD inhibition percentage with box and whiskers diagram showing all subject values. Inhibition values >30% are regarded as positive results according to the manufacturer indication. D. Percentage of subjects developing ACE2/RBD inhibition positive values (>30%) in the different groups. Kruskal-Wallis test, followed by Dunn’s post-test for multiple comparisons, was used for assessing statistical differences between groups. * P≤ 0.05; * * * P ≤ 0.001. Healthy control (HC), myelofibrosis (MF), ruxolitinib (Ruxo).
In the group of MF patients on ruxolitinib, 70% of patients showed an IgG antibody response to the spike antigen as compared to 80% of those who were not on the drug. Antibody titers began to wane at 30 days from the booster dose in controls but continued to show a rise in MF patients. This suggests a different pattern of antibody response over a longer period.
The researchers also found that in MF vaccine recipients, spike-specific antibodies elicited by the vaccine showed lower neutralization against spike binding to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell or to the receptor-binding domain (RBD) of the spike.
Almost all healthy controls showed over 30% inhibition at seven days and 30 days from the second dose. However, binding inhibition was especially low in patients treated with ruxolitinib, with less than one in five showing more than 30% inhibition at seven days and 40% at one month, compared to over half in MF subjects who were not on the drug.
Implications
The results of this study indicate that MF patients exhibit a slow antibody response following two doses of the COVID-19 mRNA vaccine. This may require this patient population to receive a third booster dose to achieve acceptable levels of protection against severe disease.
The first dose appears to be less immunogenic in these individuals, corroborating earlier studies. While 76% of these patients were seroconverted, the process was slower than in healthy controls. Over time, more of the patients on ruxolitinib developed antibodies; however, the rapid and intense rise in antibodies expected after a booster dose was conspicuously absent.
“This observation is particularly relevant for defining the vaccination schedule more fitting for these patients, suggesting a possible positive role for a third vaccine dose.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Fiorino, F., Sicuranza, A., Ciabattini, A., et al. (2021). The Slower Antibody Response in Myelofibrosis Patients After Two Doses of mRNA SARS-Cov-2 Vaccine Calls for A Third Dose. medRxiv. doi:10.1101/2021.09.21.21263627. https://www.medrxiv.org/content/10.1101/2021.09.21.21263627v1.
- Peer reviewed and published scientific report.
Fiorino, Fabio, Anna Sicuranza, Annalisa Ciabattini, Adele Santoni, Gabiria Pastore, Martina Simoncelli, Jacopo Polvere, et al. 2021. “The Slower Antibody Response in Myelofibrosis Patients after Two Doses of MRNA SARS-CoV-2 Vaccine Calls for a Third Dose.” Biomedicines 9 (10): 1480. https://doi.org/10.3390/biomedicines9101480. https://www.mdpi.com/2227-9059/9/10/1480.