Coronavirus research has shown that higher viral loads are associated with severe COVID-19 infection. But new results from the phase III Coronavirus Efficacy (COVE) clinical trial find the Moderna vaccine reduces severe acute respiratory coronavirus 2 (SARS-CoV-2) viral load and viral shedding.
In addition to lower viral loads, the vaccine decreased the risk of symptomatic infection against several coronavirus variants. For example, the two-dose Moderna vaccine provided about 80% protection against the Epsilon and Gamma variant.
The study “Initial Analysis of Viral Dynamics and Circulating Viral Variants During the mRNA-1273 Phase 3 COVE Trial” was published on the medRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Trial design
Adults without prior SARS-CoV-2 infection from 99 different locations in the United States were recruited for the phase III trial. Participants received either the Moderna vaccine or a placebo to compare their viral copy and viral shedding levels.
Once the Moderna vaccine received emergency use authorization, the trial was revised to allow people given a placebo to receive the Moderna vaccine. Thus, a second part of the trial is ongoing, and participants will be monitored for up to 2 years.
SARS-CoV-2 variants were sequenced and studied for their viral copy number and viral shedding in 799 COVID-19 cases. When the trial was revised to give participants the option for a vaccine, the researchers also decided to extend sequencing data to other respiratory pathogens.
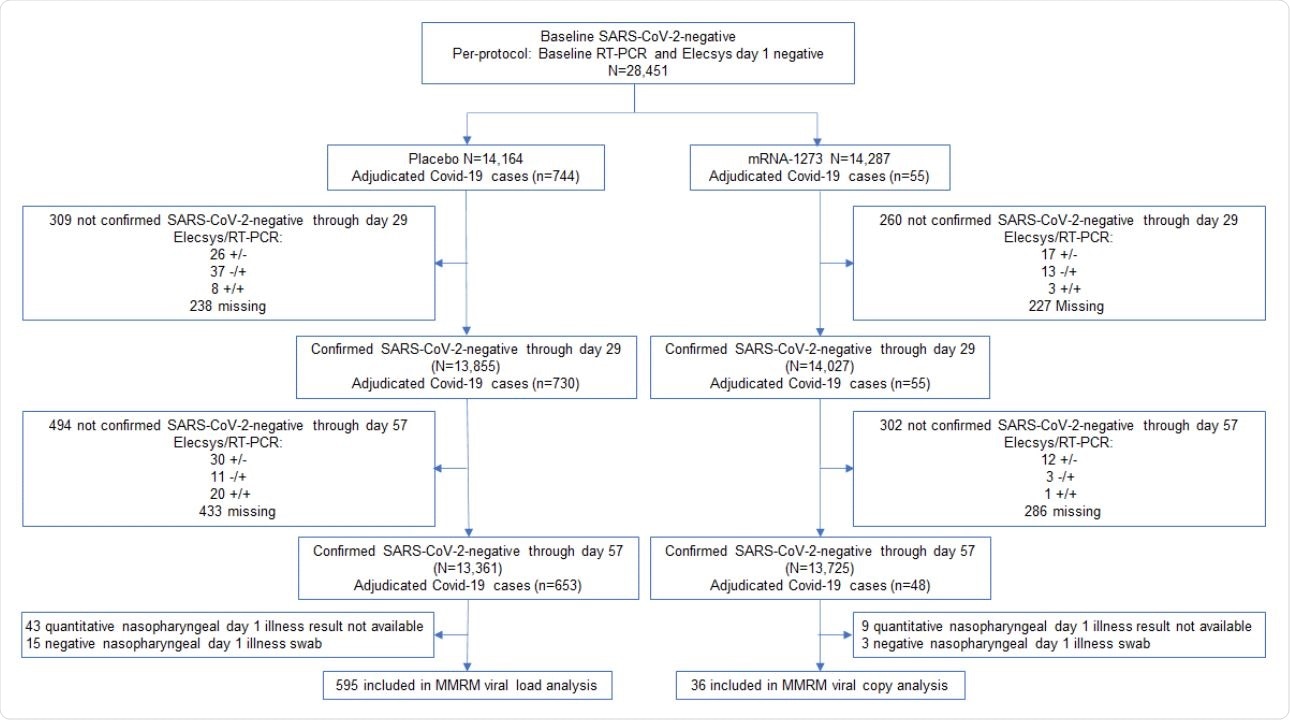
Viral copy and shedding analysis population. Included in the analysis were participants in the per-protocol population who were SARS-CoV-2 negative by both binding antibody against nucleocapsid (ROCHE Elecsys) and RT-PCR at baseline and day 29, and negative for binding antibody against nucleocapsid (ROCHE Elecsys) at day 57.1 The analysis was limited to adjudicated Covid-19 cases in the blinded portion of the study, i.e. earlier of unblinding or data cutoff date of March 26, 2021, based on a database lock which occurred on May 4, 2021.
Moderna vaccine causes a significant reduction in viral load
The Moderna vaccine was responsible for significantly decreasing SARS-CoV-2 viral copies and viral shedding in SARS-CoV-2 respiratory samples. Participants with breakthrough infections but vaccinated showed a significant reduction in viral load during diagnosis and in other saliva samples.
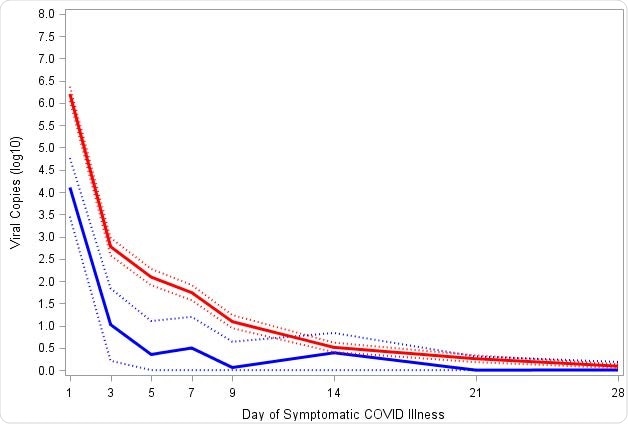
Reduction in SARS-CoV-2 viral load with mRNA-1273 compared with placebo
“Although the transmission dynamics of Covid-19 are still under study, the estimated 10- to 100-fold reductions in viral load, with statistically significant reductions in copies through day 9, may provide a significant reduction in Covid-19 disease spread, even in vaccine breakthrough cases,” explained the researchers.
As predicted, the Moderna vaccine lowered COVID-19 symptoms by reducing the risk of hospitalization, asymptomatic infections, and dying.
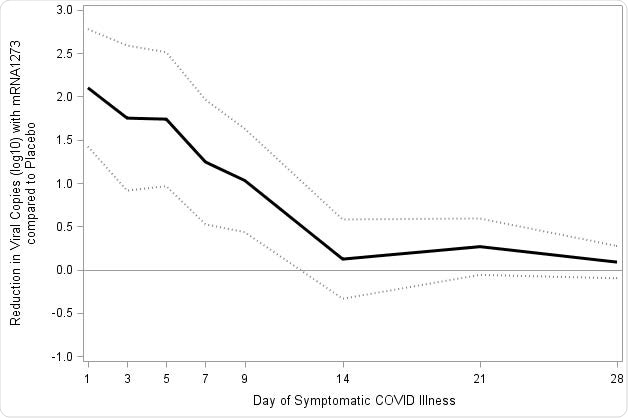
Reduction in SARS-CoV-2 viral load with mRNA-1273 compared with placebo. Viral load was assessed on the basis of SARS-CoV-2 RT-PCR cycle threshold (Ct) values converted to viral copy number as described in the methods. Mixed model repeated measures (MMRM) analysis was performed comparing absolute and change from baseline log10 viral copy between vaccinated and placebo participants based on data from nasopharyngeal swabs at day 1 of illness and saliva samples at days 3, 5, 7, 9, 14, 21, and 28 of illness. Included adjudicated cases in the blinded portion of the study. The mRNA participants (N=36) comprised 29 with first illness and 7 with second illness visits. The placebo participants (N=595) included 527 cases from first illness visits, and 61, 5, and 2 for second third and fourth illness visits respectively. A: Solid lines represent placebo (Red) and mRNA-1273 (Blue), while dotted lines correspondingly denote 95% confidence intervals. B: Difference between the mRNA-1273 and placebo participants in viral copies (log10) black solid line and 95% CI in dotted lines.
Moderna is protective against SARS-CoV-2 variants
According to sequencing SARS-CoV-2 data, some of the samples in the vaccine group and the placebo group belonged to the Epsilon, Gamma, and Zeta variants. This suggests these variants were circulating across the United States during early- to mid-2021.
“The efficacy data from this exploratory analysis of specific variants of Covid-19 cases, combined with the available respiratory pathogen data through June 2021, suggest that high vaccine efficacy was maintained during the time-frame, which encompassed not only the peak of the US epidemic (January 2021), but also the emergence of Alpha, Beta, Epsilon and Gamma variants,” explained the researchers.
The results estimate the Moderna vaccine was 82.4% effective for variants of concern and 100% effective against the Zeta variant of interest. Additionally, the Moderna vaccine was 81.2% effective against California variants B.1.427 and B.1.429.
Study limitations
The researchers note that the trial is still ongoing, and there is a possibility that future data may change initial findings. For example, the study found there was high vaccine effectiveness in preventing symptomatic infection. But with more follow-ups and potential infections from variants, the measured burden of disease and burden of infection may change.
There were also issues with sequencing SARS-CoV-2 samples. Because the vaccine lowered SARS-CoV-2 viral loads, it made it more difficult to acquire good quality sequences. As a result, there was less than 50% success rate in achieving a viral sequence in vaccine breakthrough cases, while there was an 80% success rate in the placebo group.
Capturing sequence data may also be biased. For example, the Delta variant is more infectious and may produce greater viral loads than the original SARS-CoV-2 strain. Therefore, a detection bias is possible as Delta samples are more likely to be successfully sequenced and analyzed.
Vaccine effectiveness against SARS-CoV-2 remains under investigation as the trial was amended several times. Only a small sample size of SARS-CoV-2 variants was collected and studied. Additionally, treatment groups were unblinded to allow participants the opportunity to receive the Moderna vaccine.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pajon R, et al. (2021). Initial Analysis of Viral Dynamics and Circulating Viral Variants During the mRNA-1273 Phase 3 COVE Trial. medRxiv. doi: https://doi.org/10.1101/2021.09.28.21264252, https://www.medrxiv.org/content/10.1101/2021.09.28.21264252v1
- Peer reviewed and published scientific report.
Pajon, Rolando, Yamuna D. Paila, Bethany Girard, Groves Dixon, Katherine Kacena, Lindsey R. Baden, Hana M. El Sahly, et al. 2022. “Initial Analysis of Viral Dynamics and Circulating Viral Variants during the MRNA-1273 Phase 3 COVE Trial.” Nature Medicine, February. https://doi.org/10.1038/s41591-022-01679-5. https://www.nature.com/articles/s41591-022-01679-5.